-
PDF
- Split View
-
Views
-
Cite
Cite
Neelam A. Desai, Vepatu Shankar, Single-strand-specific nucleases, FEMS Microbiology Reviews, Volume 26, Issue 5, January 2003, Pages 457–491, https://doi.org/10.1111/j.1574-6976.2003.tb00626.x
Close - Share Icon Share
Abstract
Single-strand-specific nucleases are multifunctional enzymes and widespread in distribution. Their ability to act selectively on single-stranded nucleic acids and single-stranded regions in double-stranded nucleic acids has led to their extensive application as probes for the structural determination of nucleic acids. Intracellularly, they have been implicated in recombination, repair and replication, whereas extracellular enzymes have a role in nutrition. Although more than 30 single-strand-specific nucleases from various sources have been isolated till now, only a few enzymes (S1 nuclease from Aspergillus oryzae, P1 nuclease from Penicillium citrinum and nucleases from Alteromonas espejiana, Neurospora crassa, Ustilago maydis and mung bean) have been characterized to a significant extent. Recently, some of these enzymes have been cloned, their crystal structures solved and their interactions with different substrates have been established. The detection, purification, characteristics, structure–function correlations, biological role and applications of single-strand-specific nucleases are reviewed.
1 Introduction
All living systems contain nucleases, capable of interacting with nucleic acids and hydrolyzing the phosphodiester linkages. The enzymatic breakdown of nucleic acids was first observed in the early twentieth century [1], and the term ‘nucleases’ was coined for enzymes involved in this. However, it was not until 1940 that Kunitz [2, 3] described two groups of nucleases based on sugar specificity, and subsequently different schemes of classification were proposed [3–5]. With the discovery of newer nucleases and multifunctional enzymes like micrococcal nuclease and snake-venom phosphodiesterase, however, the classification of Kunitz was found to be inadequate. Soon, a new class of sugar non-specific nucleases had to be added to the list as per new evidence. Hence, to overcome these shortcomings, Bernard [6] and Laskowski [4, 7] suggested that nucleases be classified on the basis of
- 1
the nature of substrate hydrolyzed (DNA, RNA);
- 2
the type of nucleolytic attack (exonuclease and/or endonuclease);
- 3
the nature of the hydrolytic products formed i.e. mono- or oligonucleotides terminating in a 3′- or a 5′-phosphate; and
- 4
the nature of the bond hydrolyzed.
However, these schemes of classification did not make any provision for the difference in double-stranded (ds) and single-stranded (ss) cleavage. The credit for the discovery of nucleases hydrolyzing single-stranded nucleic acids goes to Lehman [8]. With the realization of the varied and complex nature of the catalytic activities of different nucleases, it became obvious that exceptions did exist in almost every category of each of the proposed classification schemes, which led Laskowski [7] to comment that the issue of classification expired because “the progress just overgrew all boundaries”.
Single-strand-specific nucleases are ubiquitous in distribution. They exhibit high selectivity for single-stranded nucleic acids and single-stranded regions in double-stranded nucleic acids [9], and hence they are widely used as probes for the structural determination of nucleic acids, mapping mutations and studying the interactions of DNA with various intercalating agents [10]. Intracellularly, some of them have been implicated in recombination [11], repair [12] and replication [13]. Although their widespread use has led to the isolation of more than 30 single-strand-specific nucleases from various sources, only a few enzymes such as S1 nuclease from Aspergillus oryzae, P1 nuclease from Penicillium citrinum, BAL 31 nuclease from Alteromonas espejiana, Neurospora crassa, Ustilago maydis and mung bean nucleases have been characterized sufficiently. More recently, some of these enzymes have been cloned, their crystal structures solved, and their interactions with different substrates have been well established. The present review gives a comprehensive account of single-strand-specific nucleases studied to date, with special emphasis on their substrate specificity and structure–function correlations.
2 Occurrence and localization
It is well known that nucleases play an important role in the four R's, i.e. recombination, replication, restriction and repair. Moreover, extracellular enzymes have been implicated in nutrition. Hence every living organism must produce one or the other type of nuclease. Single-strand-specific nucleases have been isolated from a wide variety of sources, including microbes, plants and animals. Many of these enzymes are intracellular, but microbial enzymes like S1 nuclease [14], P1 nuclease [15] and nucleases from A. espejiana[16], Serratia marcescens[17], Thermus thermophilus HB8 [18], Anabaena sp. PCC 7120 [19] and Basidiobolus haptosporus[20] are extracellular. Bacillus subtilis 16-8S produces a single-strand-specific DNase that is associated with the cell wall membrane fraction. This enzyme is secreted into the medium in large amounts when the cells are converted to protoplasts [21]. In contrast to S1 nuclease, nuclease O from A. oryzae is found in the mycelia [14]. In the case of N. crassa[22] and Aspergillus nidulans[23], the endo-exonucleases are found in various organelles like mitochondria, vacuoles, conidia, mycelia and nuclei. Endonucleases from Streptomyces antibioticus[24] and Streptomyces glaucescens[25] are located in the periplasmic space between the cytoplasmic membrane and the cell wall, whereas the alkaline nuclease from Physarum polycephalum is located in the microplasmodia [26]. Although nucleases α[27], β[28] and γ[29] from U. maydis differ in their physicochemical properties, all of them are located intracellularly. In the case of basidiomycete fungi, such as Flammulina velutipes[30], Coprinus cinereus[31] and Lentinus edodes[32], the enzyme is located in the fruiting body. Recently, Kitamura et al. [33], using immunohistochemical techniques, demonstrated that C. cinereus endonuclease is distributed in the surface gills of the fruiting body, which contain the meiotic tissues.
In plants, single-strand-specific nucleases have been isolated from various cellular components: rye germ nuclei [34], wheat chloroplasts [35], stroma, thylakoid membrane and envelope membranes of leaf chloroplasts [36], and germinating alfalfa seeds [37]. Their presence has also been shown in the endoplasmic reticulum, Golgi apparatus, protein bodies and vacuoles of the aleurone layer of barley seeds [38], bound to chromatin in the embryo axis of germinating pea [39], cultured tobacco cells [40] and leaves of Avena [41], spinach [42] and tea [43]. Moreover, they have also been isolated from mung bean sprouts [44], germinating pea seeds [45] and barley [46], potato tubers [47] and tobacco pollen [48]. Interestingly, the nuclease from Petunia hybrida pollen was considered extracellular, since it was easily excreted into the medium during the germination of pollen grains [49].
In the case of the trypanosomes Leishmania donovani[50], Crithidia luciliae[51] and Crithidia fasciculata[52], the nuclease activity is localized on the surface membrane. Among the animals, they are found in Drosophila melanogaster[53] and various organs and organelles, such as sheep kidney [54], lamb brain [55], rat [56] and hen liver nuclei [57] and mouse mitochondria [58].
3 Detection
3.1 Agar plate method/zymogram analysis
Qualitatively, nucleases can be detected on agar plates by observing the clearance zone after precipitation of an unhydrolyzed nucleic acid–dye complex [59, 60] or by activity staining on gels following electrophoresis or isoelectric focussing [61, 62].
3.2 Detection of phosphohydrolase activities
Zlotnick and Gottlieb [63] adapted the sensitive colorimetric method of Lanzetta et al. [64] that determines Pi in the range 0.5–10 nmol, for the detection of several phosphohydrolase activities in polyacrylamide gels. This procedure [63], which results in the formation of a malachite green-phosphomolybdate complex, was used for enzymes such as acid and alkaline phosphatase, nucleotidase and ATPase. This method has an advantage over that of Abrams and Baron [65], which uses the Fiske–Subbarow reagents [66], and the procedure described by McLaughlin et al. [67], where the inorganic phosphate liberated by enzyme action is precipitated with lead nitrate.
4 Assay
4.1 Viscometry
This method is based on the decrease in viscosity of the nucleic acid samples following the action of nucleases [68].
4.2 Spectrophotometric and related methods
In general, single-strand-specific nucleases are assayed by measuring the release of acid soluble nucleotides at 260 nm, following the hydrolysis of heat-denatured DNA/RNA [3, 69]. A unit of the enzyme is defined on the basis of μmol of acid soluble nucleotides liberated [70] or μg of DNA/RNA digested [71].
The sensitivity of the assay can be increased by the use of either radio-labelled [72–74] or fluorescent-labelled substrates [75]. Alternately, nucleases can be assayed by analyzing the cleavage products following electrophoresis on either agarose [76–78] or polyacrylamide gels [79].
4.3 Atomic force microscopy
Umemura et al. [80] described the use of atomic force microscopy (AFM) for the characterization of single-strand-specific endonuclease action on linear DNA. This method has an advantage over electrophoresis and chromatography in that AFM can be used to observe individual DNA molecules, including the minor components arising from non-specific reactions. Moreover, based on AFM images, the proposed numerical method can be used to estimate the number of nicked sites per DNA molecule.
4.4 Phosphomonoesterase activity
Phosphomonoesterase activity is assayed as the inorganic phosphate liberated following the hydrolysis of either 3′- or 5′-mononucleotides. Units of enzyme activity are based on μmol inorganic phosphate liberated [81].
5 Purification
With few exceptions, the majority of nucleases are located intracellularly. Depending on the source, a crude nuclease preparation contains a unique set of contaminating proteins and hence it is difficult to postulate a general purification scheme for all enzymes. During the initial purification steps, one of the primary aims is to remove colored impurities, contributed by the pigments of the organelles (e.g. leaf, carotenoids, pancreas etc.) in the case of intracellular nucleases, or the media constituents in the case of extracellular nucleases. This is achieved by precipitation with alcohol and acetone or with ammonium sulfate. These procedures, in addition to removal of some of the contaminants, are useful in the concentration of the crude extract. Moreover, sodium chloride [82] and polyethylene glycol [83] have been used for this purpose.
Single-strand-specific nucleases are relatively thermostable enzymes and a brief exposure of the crude enzyme preparation to high temperature (60 to 70°C) has proven beneficial, as it not only helps in inactivating any protease(s) but also in removing heat-labile proteins. Although ion exchangers such as DEAE- and CM-cellulose are widely used for the purification of these enzymes, phosphocellulose has been found useful in certain cases. For example, potato tuber nuclease, despite its net negative charge at pH 7.5, binds to phosphocellulose due to affinity toward phosphate groups [47]. In this manner, this support not only acts as a cation exchanger but also as an affinity matrix. Single-strand-specific nucleases in general are relatively easy to purify to a level where they are free from contaminating nucleases and this can be achieved in one or two chromatographic steps. In the case of S1 nuclease, the most widely used enzyme, it has been shown that a single chromatographic step on DEAE-cellulose (pH 7.0) is sufficient to remove most of the contaminating nucleolytic activity [84]. Moreover, rechromatography of the partially purified enzyme preparation on DEAE-cellulose gave an enzyme preparation free of dsDNase activity [85].
Hydroxyapatite has been used extensively for the purification of single-strand-specific nucleases from carrot [86], Chlamydomonas[87], P. polycephalum[26], hen liver [57] and mouse mitochondria [58]. Furthermore, enzymes like S1 nuclease [81] and U. maydis nuclease [27] have been purified on hydrophobic matrices, such as Phenyl- and Octyl-Sepharose.
Affinity chromatography has also been employed for the purification of some of the single-strand-specific nucleases. The glycoprotein nature of nucleases from A. oryzae[81], pea seed [88] and spinach [89] have been exploited for their purification on concanavalin (Con) A-Sepharose. The preference of single-strand-specific nucleases for single-stranded nucleic acids has been utilized for the purification of S1 nuclease [90], N. crassa nuclease [91] and hen liver nuclease [57] on ssDNA bound to cellulose and Sepharose or entrapped in acrylamide. In this case, the chromatographic operation is generally carried out under conditions where the enzyme is either not active or shows very little activity. While heparin agarose was used for the purification of yeast mitochondrial [92], Schizophyllum commune[93] and barley seed [94] nucleases, Affi-gel Blue and poly(U)-Sepharose were employed for the purification of spinach nuclease [89]. Kurosawa et al. [30] used ApUp-agarose for the purification of F. velutipes nuclease, and Hb-Sepharose was used for the purification of Streptomyces tendae nuclease [95]. Gray et al. [96] purified the fast (F) form of BAL 31 nuclease on 5′-AMP–agarose. Similarly, S1 nuclease was purified using 5′-AMP–Sepharose [97]. Immunoaffinity purification with anti-S1 nuclease antibodies bound to Sepharose has also been used for the purification of S1 nuclease [98].
Modern purification techniques like HPLC and FPLC have been successfully utilized for the purification of single-strand-specific nucleases from F. velutipes[30], Penicillium sp. [99], spinach [89] and B. haptosporus[20].
6 Physical properties
6.1 Molecular mass and subunit structure
Mr of single-strand-specific nucleases are in the range 5.5–140 kDa, but the majority of them fall between 29 and 85 kDa (Table 1). The enzymes from F. velutipes[30], carrot [86] and yeast [92] are high molecular mass proteins with an Mr of 91, 100 and 140 kDa respectively. Rye germ ribosome [100] and C. cinerus[31] nucleases are comparatively low Mr proteins of 20 and 22 kDa, respectively, whereas germinating barley nuclease with an Mr of 5.5 kDa is perhaps one of the smallest enzymes reported so far [46].
Physical properties of single-strand-specific nucleases
| Enzyme | Mr (kDa) | pI | Carbohydrate content (%) | Reference |
| S1 nuclease | 32 | 4.0 | 18.00 | [97, 81] |
| P1 nuclease | 44 | 4.5 | 17.40 | [114] |
| N. crassa nucleases | ||||
| Mycelia | 55 | – | – | [109] |
| Conidia | 72 | – | – | [103] |
| BAL 31 nucleases | ||||
| Slow form (S) | 85 | 4.2 | – | [113] |
| Fast form (F) | 109 | 4.2 | – | |
| U. Maydis nucleases | ||||
| α | 55 | – | – | [27] |
| β | 75 | – | – | [28] |
| Nuclease Bh1 | 30 | 6.8 | 15 | [20] |
| Aspergillus nuclease | 28 | – | – | [23] |
| Physarum nuclease | 32 | – | – | [26] |
| SP nuclease | 43 | 7.7 | – | [89] |
| Mung bean nuclease | 39 | – | 29 | [101] |
| Wheat chloroplast nuclease | 29 | – | – | [35] |
| Rye germ ribosomes | ||||
| Nuclease I | 20 | 4.8 | 28 | [100] |
| Pea seeds nuclease | 42 | – | 20 | [45, 88] |
| Tobacco nuclease I | 35 | 5.2/5.6 | 9 | [117] |
| Alfalfa seedling nucleases | ||||
| Acid | 37 | 4.9 | – | [118] |
| Neutral | 41 | 5.3 | – | |
| SK nuclease | 52–53 | – | – | [83] |
| Hen liver nuclease | 43 | 10.2 | – | [57] |
| Rat liver nuclei nuclease | 50 | – | – | [56] |
| Mouse mitochondria nuclease | 73 | – | – | [58] |
| Enzyme | Mr (kDa) | pI | Carbohydrate content (%) | Reference |
| S1 nuclease | 32 | 4.0 | 18.00 | [97, 81] |
| P1 nuclease | 44 | 4.5 | 17.40 | [114] |
| N. crassa nucleases | ||||
| Mycelia | 55 | – | – | [109] |
| Conidia | 72 | – | – | [103] |
| BAL 31 nucleases | ||||
| Slow form (S) | 85 | 4.2 | – | [113] |
| Fast form (F) | 109 | 4.2 | – | |
| U. Maydis nucleases | ||||
| α | 55 | – | – | [27] |
| β | 75 | – | – | [28] |
| Nuclease Bh1 | 30 | 6.8 | 15 | [20] |
| Aspergillus nuclease | 28 | – | – | [23] |
| Physarum nuclease | 32 | – | – | [26] |
| SP nuclease | 43 | 7.7 | – | [89] |
| Mung bean nuclease | 39 | – | 29 | [101] |
| Wheat chloroplast nuclease | 29 | – | – | [35] |
| Rye germ ribosomes | ||||
| Nuclease I | 20 | 4.8 | 28 | [100] |
| Pea seeds nuclease | 42 | – | 20 | [45, 88] |
| Tobacco nuclease I | 35 | 5.2/5.6 | 9 | [117] |
| Alfalfa seedling nucleases | ||||
| Acid | 37 | 4.9 | – | [118] |
| Neutral | 41 | 5.3 | – | |
| SK nuclease | 52–53 | – | – | [83] |
| Hen liver nuclease | 43 | 10.2 | – | [57] |
| Rat liver nuclei nuclease | 50 | – | – | [56] |
| Mouse mitochondria nuclease | 73 | – | – | [58] |
Physical properties of single-strand-specific nucleases
| Enzyme | Mr (kDa) | pI | Carbohydrate content (%) | Reference |
| S1 nuclease | 32 | 4.0 | 18.00 | [97, 81] |
| P1 nuclease | 44 | 4.5 | 17.40 | [114] |
| N. crassa nucleases | ||||
| Mycelia | 55 | – | – | [109] |
| Conidia | 72 | – | – | [103] |
| BAL 31 nucleases | ||||
| Slow form (S) | 85 | 4.2 | – | [113] |
| Fast form (F) | 109 | 4.2 | – | |
| U. Maydis nucleases | ||||
| α | 55 | – | – | [27] |
| β | 75 | – | – | [28] |
| Nuclease Bh1 | 30 | 6.8 | 15 | [20] |
| Aspergillus nuclease | 28 | – | – | [23] |
| Physarum nuclease | 32 | – | – | [26] |
| SP nuclease | 43 | 7.7 | – | [89] |
| Mung bean nuclease | 39 | – | 29 | [101] |
| Wheat chloroplast nuclease | 29 | – | – | [35] |
| Rye germ ribosomes | ||||
| Nuclease I | 20 | 4.8 | 28 | [100] |
| Pea seeds nuclease | 42 | – | 20 | [45, 88] |
| Tobacco nuclease I | 35 | 5.2/5.6 | 9 | [117] |
| Alfalfa seedling nucleases | ||||
| Acid | 37 | 4.9 | – | [118] |
| Neutral | 41 | 5.3 | – | |
| SK nuclease | 52–53 | – | – | [83] |
| Hen liver nuclease | 43 | 10.2 | – | [57] |
| Rat liver nuclei nuclease | 50 | – | – | [56] |
| Mouse mitochondria nuclease | 73 | – | – | [58] |
| Enzyme | Mr (kDa) | pI | Carbohydrate content (%) | Reference |
| S1 nuclease | 32 | 4.0 | 18.00 | [97, 81] |
| P1 nuclease | 44 | 4.5 | 17.40 | [114] |
| N. crassa nucleases | ||||
| Mycelia | 55 | – | – | [109] |
| Conidia | 72 | – | – | [103] |
| BAL 31 nucleases | ||||
| Slow form (S) | 85 | 4.2 | – | [113] |
| Fast form (F) | 109 | 4.2 | – | |
| U. Maydis nucleases | ||||
| α | 55 | – | – | [27] |
| β | 75 | – | – | [28] |
| Nuclease Bh1 | 30 | 6.8 | 15 | [20] |
| Aspergillus nuclease | 28 | – | – | [23] |
| Physarum nuclease | 32 | – | – | [26] |
| SP nuclease | 43 | 7.7 | – | [89] |
| Mung bean nuclease | 39 | – | 29 | [101] |
| Wheat chloroplast nuclease | 29 | – | – | [35] |
| Rye germ ribosomes | ||||
| Nuclease I | 20 | 4.8 | 28 | [100] |
| Pea seeds nuclease | 42 | – | 20 | [45, 88] |
| Tobacco nuclease I | 35 | 5.2/5.6 | 9 | [117] |
| Alfalfa seedling nucleases | ||||
| Acid | 37 | 4.9 | – | [118] |
| Neutral | 41 | 5.3 | – | |
| SK nuclease | 52–53 | – | – | [83] |
| Hen liver nuclease | 43 | 10.2 | – | [57] |
| Rat liver nuclei nuclease | 50 | – | – | [56] |
| Mouse mitochondria nuclease | 73 | – | – | [58] |
Most of the single-strand-specific nucleases consist of a single polypeptide chain, but mung bean [101], pea seed [88] and C. cinerus[31] nucleases are made up of two unidentical subunits of 25 and 15 kDa, 30 and 24 kDa, and 12 and 14 kDa, respectively. Similarly, the nuclease from Aspergillus sydowii is made up of three unidentical subunits of 80, 50 and 25 kDa [102]. In contrast, the enzymes from N. crassa mitochondria [91], yeast mitochondria [92] and mouse mitochondria [58] are made up of two identical subunits of 33 kDa, 57kDa and 37.4 kDa, respectively. Mung bean nuclease showed only one band, corresponding to an Mr of 39 kDa, on SDS–PAGE, in the absence of β-mercaptoethanol but in its presence the enzyme resolved into three components, corresponding to an Mr of 39 kDa, 25 kDa and 15 kDa. Since the intact and cleaved species migrated as a single band prior to reduction, it was suggested that the cleaved species are held together by disulfide bond(s). However, both the cleaved and intact forms of the enzyme are equally active on ssDNA, RNA and 3′-AMP [101].
Interestingly, N. crassa produces four different nucleases in sorbose-containing liquid culture medium which are derived via different routes of proteolysis from a single inactive precursor polypeptide of Mr 90 kDa [103–110]. The first is a 75-kDa single-strand-specific exonuclease requiring Mg2+, identical to that from conidia but not found in mycelia. This enzyme shows 5′→3′-exonuclease activity in the presence of Mg2+, but endonuclease activity in the absence of Mg2+. The second is a 65-kDa endo-exonuclease exhibiting endonuclease activity on ssDNA but exonuclease activity on dsDNA. The third, a 55-kDa single-strand-specific endonuclease, is identical to that originally isolated from the mycelia by Linn and Lehman [111, 112]. The fourth enzyme, secreted by mycelia, is a 65-kDa Ca2+-dependent endonuclease which cleaves both ss- and dsDNA but has no RNase activity. In addition, single-strand binding endo-exonuclease exhibiting high ssDNase activity of Mr 31–33 kDa, have been purified from mitochondria, vacuoles and a mixture of these organelles [91]. The extracellular nuclease from A. espejiana sp. BAL 31 has been isolated as two distinct proteins, the ‘fast’ (F) and ‘slow’ (S) species, with an Mr of 109 and 85 kDa, respectively [113].
6.2 Isoelectric point
The pI values of single-strand-specific nucleases are in the range 4.0–10.2. P1 [114], S1 [115], BAL 31 [113] and rye germ ribosome [100] nucleases are acidic proteins, having a pI of 4.5, 4.3, 4.2 and 4.8, respectively. Spinach nuclease is a basic protein with a pI of 7.7±0.3 [89]. However, the enzyme from hen liver nuclei is a highly basic protein with a pI of 10.2±0.2 [57]. Highly purified preparations of S1 nuclease showed one major band and two minor forms corresponding to a pI of 3.67, 3.35 and 3.53, respectively [81], while crude S1 nuclease showed a single band corresponding to a pI of 4.3 [115]. The formation of multiple forms of the purified enzyme was attributed to the partial degradation of the enzyme during its purification from commercial Takadiastase powder or due to heat treatment at 70°C, during the purification step [116]. Although nuclease I from Nicotiana tabacum is a monomer of 35 kDa, two forms of the enzyme with pI of 5.2 and 5.6 could be resolved by electrofocussing. These forms did not exhibit any significant difference in their catalytic properties [117]. In contrast, acid and neutral nucleases from alfalfa seeds, although exhibiting different pH optima, had pI values in the acidic range (4.9 and 5.3, respectively) [118].
6.3 Glycoprotein nature
Some of the well-studied single-strand-specific nucleases like P1 [114], S1 [81], mung bean [101], pea seed [88], barley [94] and rye germ ribosome nucleases [100], a nuclease from Penicillium sp. [99], spinach nuclease [89] and nuclease Bh1 [20] are glycoproteins and their carbohydrate content varies from 15–29%. Compared to these nucleases, tobacco nuclease I has a very low carbohydrate content (9%). Preliminary studies on the carbohydrate moiety of P1 nuclease revealed that it consists of mannose, galactose and glucosamine in a ratio of 6:2:1 [114]. Rye germ ribosome nuclease contains 28% carbohydrate and the carbohydrate moiety was shown to contain fucose, mannose and glucosamine [100]. In the case of S1 nuclease, out of two carbohydrate moieties, one of them is a high mannose type [119]. Glycoproteins are known to exhibit anomalous behavior on gel filtration and SDS–PAGE, leading to incorrect estimation of their Mr[120]. Nucleases PA1, PA2 and PA3 from Penicillium sp. showed an Mr of 35 kDa, 33 kDa and 32 kDa, respectively, on SDS–PAGE [99]. Since the amino acid composition of all the species were very similar, it was concluded that the difference in Mr of these enzymes was due to differential glycosylation. Similarly, nuclease Bh1 showed an Mr of 41 kDa by gel filtration, but on SDS–PAGE gave two bands of Mr 37 and 32 kDa. However, on deglycosylation it showed a single band with an Mr of 30 kDa. Since the partial N-terminal sequence of both 37- and 32-kDa proteins were identical, it was concluded that the two bands on SDS–PAGE were due to differential glycosylation [20]. Trimble and Maley [120] attributed the difference in the Mr of native and deglycosylated forms of P1 and mung bean nucleases to the carbohydrate moiety. Most glycoproteins are also known to be resistant to the action of proteases [121]. This is supported by the observation that pea seed nuclease, after treatment with trypsin for 1 h, lost only 30% of its initial activity, whereas DNase I, which is a non-glycosylated protein, was inactivated completely within 10 min of trypsin digestion [88].
7 Catalytic properties
7.1 Optimum pH and pH stability
The optimum pH of a nuclease is an important criterion that determines its potential as an analytical tool. Experiments on nucleic acids are best done at or around neutral pH. The pH optima of single-strand-specific nucleases range from 4–9. Some of the widely used and well-studied enzymes like S1, P1 and mung bean nucleases have acid pH optima in the range 4.0–5.0. Having an acid pH optimum is disadvantageous since lower pH values lead to considerable depurination of DNA. In contrast, nucleases from Aspergillus sojae[122], B. subtilis[123], T. thermophilus[18] and Proteus mirabilis[124] have pH optima on the alkaline side in the range 9–10. Although S1 nuclease exhibited an acidic pH optimum, the intracellular nuclease O exhibited a broad pH optimum of 7.2 to 8.2. Most of the enzymes exhibit the same pH optimum for the hydrolysis of both monomeric and polymeric substrates [125]. However, enzymes like BAL 31 nuclease [96], N. crassa (mitochondria) nuclease [126] and U. maydis nuclease α[27] showed different pH optima for the hydrolysis of ssDNA (8.8, 6.5–7.5 and 8.0) and dsDNA (8.0, 5.5–6.5 and 5.0), respectively. 3′-Nucleotidase-nuclease from potato tubers [47] showed different pH optima for nucleotidase (pH 8.0) and nuclease (pH 6.5–7.5) activities, whereas wheat chloroplast nuclease showed an optimum pH of 7.8 and 6.8 for the hydrolysis of denatured DNA and RNA, respectively [35]. Similarly, tobacco nuclease hydrolyzed ssDNA and RNA optimally at pH 5.2 to 6.0, but the phosphomonoesterase activity was optimal at pH 7.0 [40]. Nucleases Le1 and Le3 from L. edodes [32, 127] and P1 nuclease from P. citrinum[70] showed different pH optima for the hydrolysis of different mononucleotides. In contrast, S1 nuclease [128] and nuclease Bh1 [20, 129, 130] exhibited the same pH optimum for the hydrolysis of both monomeric and polymeric substrates. S. tendae was active over a broad range pH (4.5–10.5) when assayed with ssDNA [95]. Yupsanis et al. [37] isolated two nucleases from alfalfa seeds with optimum pH values of 5.5 and 7.0. P1 nuclease from P. citrinum was stable between pH 5 and 8 [70], whereas the nuclease from A. sydowii was stable in the pH range 5–9 [102]. Nuclease Bh1 showed high stability and retained its full activity for 24 h at pH 7.0 and 37°C [20, 129, 130].
The optimum pH of some of the nucleases is also dependent on factors such as ionic strength and the presence of metal ions. For example, the optimum pH of the endonuclease from P. polycephalum[26] increased from 7.0 to 8.5 with an increase in ionic strength of the buffer, whereas nuclease γ from U. maydis[29] showed an optimum pH of 8.0 and 9.0 in presence of Mg2+ and Mn2+, respectively.
7.2 Optimum temperature and temperature stability
The temperature optima of most of the well-characterized single-strand-specific nucleases are in the range 37–70°C [125]. Increase in the temperature, from 47–62°C, did not significantly affect the rate of reaction of N. crassa[111] and spinach [89] nucleases. S1 nuclease, however, showed two- and three-fold increase in the activity on ssDNA at 45 and 60°C, respectively, than at 35 and 37°C [68, 131]. Pea seed nuclease exhibited an optimum temperature of 45°C for nuclease activity and 60°C for phosphomonoesterase activity [88]. However, nuclease Bh1 showed the same optimum temperature (60°C) for its ssDNase, RNase and phosphomonoesterase activities [20, 129, 130]. A nuclease from T. thermophilus is perhaps the only enzyme having a very high temperature optimum of 85°C [18].
Single-strand-specific nucleases, in general, are thermostable enzymes and in the case of some of the well-characterized enzymes, like S1 nuclease [132], P1 nuclease [114] and mung bean nuclease [101], the increased thermal stability has been attributed to the presence of a high amount of hydrophobic amino acids.
7.3 Metal ion requirement
Most of the single-strand-specific nucleases, with the exception of F. velutipes[30], tobacco [40] and barley [46] nucleases and nuclease β from U. maydis[28], are either metalloenzymes or metal-requiring enzymes [125]. S1 [132], P1 [114], mung bean [133] and P. polycephalum nucleases [26], nuclease PA3 from Penicillium sp. [99], nuclease Bh1 [134] and a 3′-nucleotidase-nuclease from C. luciliae[135] are zinc metalloproteins, while the enzyme from N. crassa is a cobalt metalloprotein [109]. S. commune nuclease is either a zinc or cobalt metalloprotein [93], whereas the enzyme from A. sydowii is a calcium and magnesium metalloprotein [136]. S1 nuclease, the extracellular enzyme from A. oryzae, showed a requirement of Zn2+ for activity [137], whereas the intracellular nuclease O required Mg2+ for its activity [14]. Although nuclease α from U. maydis[27] and wheat chloroplast nuclease [35] did not require metal ions for their activity, nuclease α was stimulated four-fold by Co2+, whereas the ssDNase activity of wheat chloroplast nuclease showed only slight stimulation (20%) in the presence of Mg2+. Similarly, the 3′-nucleotidase activity of pea seed nuclease did not show an obligate requirement of metal ions for its activity but was stimulated approximately two-fold in presence of MgCl2 and CaCl2[88]. The acid and neutral nucleases from alfalfa seeds also did not require metal ions for their activity but exhibited differential sensitivity towards metal ions. Thus the acid nuclease was highly stimulated by Zn2+, whereas the neutral nuclease was strongly inhibited in its presence. Similarly, Mn2+ and Ni2+ stimulated the acid nuclease slightly but brought about approximately 50% inhibition of the neutral nuclease [37]. S1 nuclease shows an optimum pH of 4.0 to 4.5 and requires either Zn2+ or Co2+ for its optimal activity. Additionally, it shows very little activity at pH 7.0. However, Esteban et al. [138] demonstrated that, in the presence of high concentration of Mg2+ (20mM), S1 nuclease could degrade ssDNA at pH 7.5 and the pattern was similar to the one observed at pH 5.0 with 1 mM Zn2+. Nucleases from yeast [92], mouse mitochondria [58] and Actinomyces sp. [139] require Mg2+ for their optimal activity, whereas the enzymes from Chlamydomonas[87] and B. subtilis need Ca2+ for their optimal activity [140]. A. sydowii nuclease was optimally active in the presence of 20 mM Mg2+, 0.4 mM Mn2+ or 2 mM Co2+. The relative activities of this enzyme in the presence of optimum concentrations of Mg2+, Mn2+ or Co2+ were 100%, 14% and 8%, respectively [102]. However, nuclease Bh1 from B. haptosporus neither showed the requirement of metal ions for its activity nor was the activity stimulated in the presence of metal ions. Moreover, all the activities, namely ssDNase, RNase and 3′-nucleotidase, were inhibited by Ag2+, Hg2+, Zn2+, Fe3+ and Al3+ [20, 129, 130].
Some of these enzymes require more than one divalent cation for their optimal activity. Like N. crassa[141] and U. maydis[142] nucleases, A. nidulans[23] nuclease requires three divalent cations, Mg2+, Mn2+ and Zn2+, for maximum activity. D. melanogaster nuclease requires Mg2+ and Mn2+[53] while the enzyme from carrot shows maximum activity in presence of Mg2+, Mn2+, Ca2+ and Zn2+[143]. The action of N. crassa nuclease on dsDNA is dependent on the Mg2+ concentration, but its activity on ssDNA is not, although it is stimulated to some extent [109]. Moreover, the pH optimum of N. crassa nuclease for the hydrolysis of dsDNA and RNA varies with Mg2+ concentration [103]. The addition of 10 mM of Mg2+, Ca2+ or Fe2+ resulted in 2.5-fold stimulation of the ssDNase activity of the N. crassa enzyme, but it also brought about approximately 40% inhibition of RNase activity. The selective inhibition of the RNase activity in the presence of metal ions was correlated with the probable induction of secondary structures in RNA by these metal ions. On the other hand, Co2+, which appears to be a cofactor of the enzyme, stimulated its activity three-fold towards all the substrates [141]. The meiotic nuclease I from C. cinereus needs Mg2+ and/or Ca2+ as co-factors [31]. While Ca2+ is more efficient than Mg2+, the enzyme shows maximum activity when both the cations are used in combination. Meiotic endonucleases II and III require Mg2+ as a cofactor but for meiotic endonuclease III, Ca2+ can also function as a co-factor [144, 33].
7.4 Stability to denaturants
S1 nuclease [71] and barley nuclease [46] are stable to low concentrations of denaturants like SDS and/or urea. Though P1 nuclease is susceptible to guanidine hydrochloride and SDS, the inhibition of the enzyme by urea and guanidine hydrochloride is reversible [9]. Gray et al. [16] showed that the S form of BAL 31 nuclease is active in the presence of 5% (w/v) SDS and can be incubated with the detergent without loss of activity if Ca2+ and Mg2+ are present at a concentration of 12.5 mM before the addition of the detergent. Purified S form of the BAL 31 nuclease retained approximately 60% of its maximal activity in the presence of 4 M urea [96], whereas nuclease Bh1 retained 80% of its ssDNase, RNase and 3′-nucleotidase activities in 4 M urea [20, 129, 130]. The nuclease from A. sydowii was completely inactivated in 3 M urea due to the dissociation of the protein into subunits. However, the inactivation with urea was completely reversible in presence of 10 mM Ca2+[136]. In contrast, P. polycephalum nuclease was stable in presence of 5 M urea [145].
7.5 Effect of organic solvents
Organic solvents like formamide, dimethylformamide, dimethylsulfoxide and glyoxal interact with DNA and reduce its overall stability. Formaldehyde [146] and glyoxal [147] bring about chemical modification of the nucleotides in unpaired strands of DNA. Formaldehyde has been widely used to prevent interstrand renaturation [148]. Isolation of single-strand-specific nucleases exhibiting high stability in the presence of organic solvents have added a new dimension to these studies as they can be used as probes for the determination of the secondary structure of DNA in the presence of various organic solvents. For example, the use of formamide has enabled the visualization, via electron microscopy, of non-bushed single-stranded regions in DNA [149, 150]. S1 nuclease showed high stability in 60% (v/v) formamide, 30% (v/v) dimethylformamide, 50% (v/v) dimethylsulfoxide and 2% (v/v) formaldehyde [151]. Moreover, Case and Baker [152] showed that S1 nuclease exhibits high stability in the presence of 100–250 mM glyoxal and hence could also be used to obtain thermal-melting profiles in the presence of formamide. Similarly, Muhich and Simpson [153] demonstrated that mung bean nuclease can linearize kDNA minicircles, from trypanosomes, in 40–50% (v/v) formamide. However, nuclease Bh1 retained its full activity in 50% (v/v) formamide but was stable only in the presence of low concentrations (10%v/v) of dimethylformamide and dimethylsulfoxide [20]. Nucleases from S. glaucescens[154] and S. antibioticus[155] exhibited more than two-fold stimulation of their activity in the presence of dimethylsulfoxide.
7.6 Effect of salt concentration
Salt concentration in the reaction mixture can affect the activity of single-strand-specific nucleases. For example, the activity of BAL 31 nuclease is maximum in the range 0–2 M NaCl and the enzyme shows only 40% of its activity in 4.4 M NaCl [96]. While 100–200 mM NaCl completely inhibited the dsDNase activity of N. crassa nuclease, it had only a marginal effect on the ssDNase activity [109]. Similarly, in the case of D. melanogaster nuclease, 30 mM NaCl inhibited 50% of the dsDNase activity whereas it required 100 mM NaCl to bring about the same level of inhibition of the ssDNase activity [53]. The inhibition of the dsDNase activity in high salt concentrations was correlated with the suppression of localized melting by electrostatic stabilization of the DNA, especially the stabilization of AT regions [156, 157]. S1 nuclease, on the other hand, is optimally active at 100 mM NaCl. The enzyme is relatively insensitive to salt concentrations between 10 and 200 mM NaCl, and in 400 mM NaCl it degrades ssDNA at 55% of the maximal rate. The stringency of S1 nuclease is maximum at high salt concentrations [71]. In contrast, NaCl inhibited P. polycephalum nuclease [26], while both KCl and NaCl inhibited mouse mitochondrial nuclease [58]. Mung bean [158] and Actinomyces sp. [139] nucleases are optimally active in the range 20–50 mM NaCl, but spinach nuclease requires 50–75 mM NaCl for maximal activity [89]. Action of rye germ nuclei nuclease, on PM2 DNA, showed that it is strongly dependent on salt concentration, but the presence of high salt (>100 mM) results in a significant inhibition of the activity [34]. Similarly, the action of S1 nuclease, on PM2 DNA, was found to be more specific at NaCl concentrations greater than 200 mM [159]. Sodium chloride in the range 50–150 mM completely inhibited the enzyme from sheep kidney [83]. Chlamydomonas nuclease showed significant inhibition in the presence of 10 mM NaCl but KCl at this concentration had no effect on the enzyme activity [87]. Nuclease Bh1 was optimally active between 25 and 50 mM of NaCl or KCl but an increase in salt concentration brought about a progressive decrease in the activities [20, 129].
7.7 Inducers, activators and inhibitors
As mentioned earlier, the key roles of nucleases are in replication and recombination processes and hence many of these enzymes are produced constitutively. Optimization of the growth conditions can enhance their levels. However, in the case of barley nuclease it was observed that gibberellic acid brought about an eight-fold increase in the de novo synthesis of the enzyme in aleurone layers [94]. The trypanosome C. luciliae[135] is incapable of de novo purine synthesis and produces a 3′-nucleotidase-nuclease which provides purine nucleosides to these parasites. The enzyme activity increases up to 1000-fold when the organism is maintained in a medium depleted of purines and/or inorganic phosphate. Moreover, cycloheximide (a protein synthesis inhibitor) and actinomycin D (a RNA synthesis inhibitor) inhibited the enzyme synthesis.
Polyamines such as spermine and spermidine, which bind to double-stranded nucleic acids, also inhibit the ssDNase activity of nucleases. Spermine stimulated the exonuclease activity of BAL 31 nuclease, but the cleavage specificity of both BAL 31 and S1 nuclease was considerably reduced in its presence [160]. Spermidine stimulated the RNase activity of yeast mitochondrial nuclease [161], whereas it had no effect on the endonuclease from S. glaucescens[154]. Both the acid and neutral nucleases from alfalfa seeds showed similar sensitivity to polyamines and the inhibition was in the order spermine>spermidine>putrescine. In the case of nuclease I from rye germ ribosomes, it was noted that low concentrations (0.1 mM) of polyamines such as putrescine and spermidine inhibited ribonuclease activity, whereas higher concentrations (2.5 mM) had a stimulatory effect [100]. Similar observations were made with Staphylococcal nuclease [162]. Thus, at low concentrations, polyamines act by changing the electrostatic potential of the enzyme–substrate complex and participate in the regulation of nucleic acid levels in cells by controlling nuclease activity [163].
Most of the single-strand-specific nucleases are either metalloenzymes or metal requiring enzymes and hence they are strongly inhibited by metal chelators like EDTA, EGTA, citrate and 8-hydroxyquinoline. While 8-hydroxyquinoline inhibited pea seed nuclease, EDTA had no effect [45]. However, its 3′-nucleotidase activity was strongly inhibited by EDTA [88]. Similarly, the ssDNase activity of wheat chloroplast nuclease was strongly inhibited by EDTA, but it had no significant effect on the RNase activity [35]. In contrast, nuclease α from U. maydis[27] was inhibited by EDTA and β-mercaptoethanol, while nuclease β from U. maydis[28] was insensitive to EDTA and 1,10-phenanthroline and reducing agents like DTT and β-mercaptoethanol. Metal ions like Mn2+, Co2+ and Zn2+ inhibited the nucleases from potato tubers [47] and B. subtilis[140]. Sheep kidney nuclease was inhibited by p-chloromercuribenzoate [83], whereas HgCl2 and CoCl2 inhibited pea seed nuclease [88]. Anions such as chloride, phosphate, succinate, bromide, carbonate, oxalate, propionate and sulfate activated the endonuclease from S. commune, while fluoride, pyrophosphate, citrate, poly(vinyl sulfate) and inorganic phosphate strongly inhibited the enzyme activity [93]. Neither divalent cations nor metal chelators affected the 5′-nucleotidase activity of L. donavani nuclease, whereas EDTA inhibited its 3′-nucleotidase activity. Moreover, compared to the 3′-nucleotidase activity, the 5′-nucleotidase activity of the enzyme was strongly inhibited by fluoride, tartarate and molybdate [164]. However, metal chelators like EDTA, EGTA, 8-hydroxyquinoline and citrate and anions such as phosphate and pyrophosphate inhibited all three activities of nuclease Bh1 [20, 129, 130]. Netropsin, a bactericidal and antiviral compound, was found to enhance the single-strand-specific endonuclease activity of BAL 31 nuclease but inhibited its exonuclease activity [165]. Netropsin selectively interacts with the AT-rich sequences in dsDNA and can induce the reversal from Z-form and other non-B-forms to the B-form of DNA. It also enhances the susceptibility of negatively superhelical DNA to S1 nuclease, but increasing the concentration of intercalating agents like ethidium bromide, adriamycin and actinomycin-D and DNA binding substances such as proflavine, chartreusin and chromoycin inhibited its activity [166].
Endo-exonuclease from N. crassa (mycelia) was inhibited by a heat-stable, trypsin-sensitive, cytosolic 24-kDa polypeptide. The protein inhibited the ssDNase activity non-competitively but the dsDNase activity was inhibited competitively. In addition, the inhibitor blocked the formation of site-specific double-stranded breaks and nicking of linearized pBR322 DNA. It also inhibited the RNase activity of N. crassa nuclease as well as the immunochemically related nuclease from A. nidulans[167]. Similarly, a heat-stable protein found in the fresh mycelia of A. oryzae inhibited the intracellular nuclease O but failed to inhibit the extracellular enzymes like S1 nuclease and RNases T1 and T2. This polypeptide inhibitor was reported to regulate the intracellular levels of nuclease during the active growth phase of A. oryzae[168]. The endonuclease NucA from Anabaena sp. was inhibited by its polypeptide inhibitor NuiA, while the related Serratia nuclease was not inhibited by a 10-fold excess of the inhibitor [169]. Cleavage of the monomeric substrate 3′, 5′-bis-(p-nitrophenyl phosphate) by NucA, however, was not inhibited by NuiA, suggesting that small molecules gain access to the active site of NucA in the enzyme-inhibitor complex under conditions where cleavage of DNA is completely inhibited [170].
The hydrolysis of polymeric substrates by single-strand-specific nucleases shows autoretardation due to end-product inhibition. S1 nuclease was inhibited competitively by 5′ribo- and deoxyribonucleotides, with deoxyribonucleotides being the more potent inhibitors [126]. Similarly, nuclease β from U. maydis was inhibited by 3′-nucleotides [28]. On the contrary, nuclease Bh1 was inhibited only by guanosine 5′-nucleotides [20, 129, 130]. Interestingly, 1–5 mM ATP inhibited the nicking activity of meiotic nuclease III from C. cinereus only in the presence of Ca2+ but was ineffective in the presence of Mg2+[33]. In addition to mononucleotides, the 3′-nucleotidase activity of S1 nuclease [128] and nuclease Bh1 [130] and that of 3′-nucleotidase-nuclease from potato tubers [171] was inhibited by polymeric substrates like ssDNA, ssDNA and RNA and poly A, respectively. In contrast, the 3′-nucleotidase activity of pea seed nuclease was stimulated 4.5-fold in the presence of native DNA, whereas with denatured DNA the stimulation was only 2.6-fold, suggesting that DNA acts as a positive modulator of the nucleotidase activity [88].
8 Substrate specificity
Although several nucleases that act on single-stranded nucleic acids have been reported to date, it is difficult to clearly demarcate between strict single-strand-specific nucleases, single-strand-preferential nucleases and those which cleave both single- and double-stranded nucleic acids with equal efficiency. This is because any enzyme from the aforementioned category can act on a variety of substrates under different experimental conditions. Despite this, single-strand-specific nucleases, owing to their high specificity for single-stranded nucleic acids, have formed a distinct group of enzymes. They are sugar non-specific, multifunctional enzymes and exhibit high selectivity for ssDNA and RNA. Some of them also show 3′- or 5′-phosphomonoesterase activity. However, the rate of hydrolysis of these substrates varies, depending on the source of the enzyme. Thus, S1 [81], mung bean [172] and tobacco [40] nucleases prefer ssDNA to RNA and 3′-AMP, whereas P1 [70], PA3 [99], Le1 [32], Le3 [127] and potato tuber [47] nucleases show higher activity on 3′AMP and RNA. The substrate specificity of P1 nuclease falls in the order of 3′AMP>RNA>ssDNA≫dsDNA [70], while that of tobacco nuclease is ssDNA>3′AMP>RNA>dsDNA [40]. However, nuclease Bh1 hydrolyzed various substrates in the order ssDNA≃3′AMP≫RNA>ds DNA [20]. Similarly, the 3′-nucleotidase-nuclease from C. luciliae hydrolyzed RNA faster than ssDNA with no detectable hydrolysis of dsDNA [135]. In contrast to the majority of plant nucleases, which prefer RNA to DNA, the acid and neutral nucleases from alfalfa seeds preferred ssDNA to RNA and hydrolyzed these substrates in the order ssDNA>RNA>dsDNA and they also exhibited 3′-nucleotidase activity [37]. Many of these enzymes are also capable of hydrolyzing double-stranded nucleic acids, though at high enzyme concentrations. A comparative study of nucleases exhibiting high selectivity for single-stranded nucleic acids, based on their ssDNase:dsDNase activity ratios and kinetic constants, indicated that mung bean nuclease has the highest preference for ssDNA (30 000), followed by S1 (10 000), S. commune (20–5000), N. crassa (250–4000), P1 (250) and U. madyis (200) nucleases [173] and nucleaseBh1 (250–300) [20]. Nucleases α[28] and β[28] from U. maydis showed high activity on ssDNA, while nuclease γ preferred dsDNA as a substrate [29].
The nuclease from Staphylococcus aureus[174] hydrolyzes both DNA and RNA but has greater affinity for DNA. Although, the activity on denatured DNA is greater than on native DNA, the single-strand specificity of the enzyme is not very high. Nuclease Rsn from Rhizopus stolonifer hydrolyzes various substrates in the order ssDNA>dsDNA≫RNA [175], and hence can be classified as a single-strand-preferential enzyme because it shows higher activity on ssDNA. Moreover, the ratio of ssDNase:dsDNase activity varied with the type of metal ion used in the reaction mixture and the enzyme exhibited approximately 1.66, 1.75 and 4.50-fold higher activity on ssDNA in the presence of Mg2+, Mn2+ and Co2+, respectively. Similarly, endonuclease M from the kinetoplasts of the protozoan parasite L. donovani hydrolyzed ssDNA two-fold faster than dsDNA suggesting it to be a single-strand-preferential enzyme. Moreover, the enzyme degraded single-stranded RNA rapidly but the RNA:DNA hybrids were resistant to cleavage. With increasing concentrations of EndoM, the unlabelled single-stranded overhang of DNA from the RNA:DNA hybrid was cleaved to give the perfect dsRNA–DNA hybrid. However, in presence of 10-fold excess enzyme, the resulting RNA:DNA hybrid was also cleaved [176]. Wheat seedling nuclease [177] that acts on ssDNA, RNA and 3′-AMP and yeast nuclease [82] that acts only on ssDNA and RNA showed the same rate of hydrolysis for all the substrates. Additionally, other well-studied sugar non-specific nucleases from S. marcescens [178, 179], Anabaena[19], Syncephalastrum racemosum[180] and Saccharomyces cerevisiae[181] hydrolyzed ssDNA, dsDNA and RNA at similar rates.
9 Mode of action
Although single-strand-specific nucleases recognize and hydrolyze a wide spectrum of substrates, they primarily cleave the internucleotide phosphodiester linkage. Based on the requirement of a free terminus, these enzymes can be classified as:
9.1 Endonucleases
They attack the internal phosphodiester bonds of nucleic acids with or without free termini. Endonucleases can also act on covalently closed circular DNA. They show a distributive mode of action and the products of hydrolysis are oligonucleotides and/or mononucleotides.
9.2 Exonucleases
These enzymes require a free terminus for their action and are incapable of hydrolyzing covalently closed circular substrates. The products of hydrolysis are predominantly mononucleotides and the mode of attack is processive.
9.3 Endo-exonucleases
This group of enzymes exhibit both exo- and endo-mode of action.
Although single-strand-specific nucleases hydrolyze both DNA and RNA either endonucleolytically or exonucleolytically, some enzymes exhibit different modes of action on these substrates. For example, nucleases from wheat chloroplasts [35], wheat chloroplast stromal protein [36], rye germ ribosomes [100], nucleoplasm of rye germ nuclei [34], barley [94] and yeast [82] hydrolyze ssDNA and RNA endonucleolytically. In contrast, A. sydowii nuclease degrades both DNA and RNA exonucleolytically in 3′→5′ direction [102], whereas B. subtilis enzyme cleaves DNA exonucleolytically from the 5′-end [182]. However, wheat seedling nuclease exhibits endonuclease activity towards ssDNA but exonuclease activity towards RNA [183]. Similarly, nuclease from F. velutipes exhibits endonucleolytic activity on ss- and dsDNA but RNA and linear polynucleotides are degraded exonucleolytically [184]. Meiotic nuclease I from C. cinereus is strictly an endonuclease [31], whereas meiotic nuclease II exhibits single-strand-specific endonuclease as well as an exonuclease activity on ssDNA [144]. Nuclease β from U. maydis exhibits both endo- and exo-mode of action on DNA. The high proportion of mononucleotides in the initial stages of hydrolysis of ssDNA by nuclease β is indicative of an exo-mode of action. However, it hydrolyzes ssDNA in a distributive manner, suggesting an endo-mode of action. Moreover, the enzyme hydrolyzes linear DNA in an exo fashion from the 5′-end [28]. Nuclease α from U. maydis[27] and BAL 31 nuclease [96], on the other hand, hydrolyze ssDNA endonucleolytically and shorten the linear duplex DNA from both 3′- and 5′-ends. In contrast to U. maydis nucleases α and β, nuclease γ does not exhibit any exonucleolytic activity on DNA [29]. As mentioned earlier, N. crassa produces four major nucleases and all of them exhibit different modes of action. The 75-kDa nuclease exhibits a 5′→3′-exonuclease activity on DNA in the presence of Mg2+ but, in the absence of Mg2+, cleaves DNA endonucleolytically. The 65-kDa endo-exonuclease exhibits endonuclease activity towards ssDNA but exonuclease activity towards dsDNA, whereas the 55-kDa product cleaves ssDNA endonucleolytically. However, the enzyme isolated from N. crassa mitochondria shows distributive endonuclease activity towards ssDNA but processive exonuclease activity towards dsDNA [91].
The end products of hydrolysis of DNA and RNA by single-strand-specific nucleases are 5′- or 3′-mononucleotides and/or oligonucleotides terminating in 5′- or 3′-phosphoryl termini. However, the same enzyme does not produce both 5′- and 3′-phosphorylated end products. S1 [137, 81], P1 [185], N. crassa[109], Bh1 [20], mung bean [101] and wheat seedling [183] nucleases produce 5′-mononucleotides as the end products of DNA and RNA hydrolysis. The oligonucleotides produced in the initial stages of hydrolysis by these enzymes have 3′-OH and 5′-PO4 termini. In contrast, nuclease β from U. maydis hydrolyzes ssDNA and RNA liberating 3′-mononucleotides [28]. Although BAL 31 nuclease [96] and nuclease α from U. maydis[27] hydrolyze linear duplex DNA from both 3′- and 5′-PO4 termini, the end products of hydrolysis are 5′-mononucleotides. Wheat chloroplast nuclease hydrolyzes ssDNA endonucleolytically, liberating oligonucleotides with 3′-OH and 5′-PO4 termini while oligonucleotides from RNA hydrolysis have 3′-PO4 and 5′-OH termini [35]. Rye germ ribosome nuclease, on the other hand, liberates oligonucleotides ending in 3′-OH and 5′-PO4 from RNA and 3′-PO4 and 5′-OH from ssDNA [36]. The end products of poly(A) or synthetic deoxyoligonucleotide hydrolysis by the acid nuclease from alfalfa seeds are 3′-mononucleotides and oligonucleotides terminating in 3′-PO4, whereas those of the neutral nuclease contain only oligonucleotides with 5′-PO4 termini. Nuclease from S. commune acts on DNA endonucleolytically to produce dinucleotides bearing 5′-PO4 termini [93]. The endonucleolytic cleavage of ssDNA by meiotic nuclease II generates oligonucleotides with 3′-PO4 termini and these oligonucleotides are resistant to the associated exonuclease activity of the enzyme. However, after removal of the 3′-PO4 with alkaline phosphatase, the exonuclease activity of the enzyme degrades the linear ssDNA in the 3′→5′ direction, generating 5′-mononucleotides. Hence, the authors proposed that the endonuclease activity is responsible for generating single-stranded nicks and/or double-stranded breaks which will not be further degraded by the exonuclease activity, so that such single-stranded nicks and double-stranded breaks can participate as substrates in the subsequent recombination events. This was correlated to the appearance of high levels of nuclease activity in meiotic prophase [144].
10 Conformational specificity
10.1 Action on polynucleotides
Action of single-strand-specific nucleases on synthetic polynucleotides revealed that the rate of hydrolysis varies with the source of the enzyme and is strongly pH dependent. Fujimoto et al. [185] noted that P1 nuclease could readily hydrolyze poly(A) and poly(C) at pH 6.0 but these substrates were highly resistant to enzymatic attack at pH 4.5. On the contrary, poly(U) and poly(I) were hydrolyzed rapidly at pH 4.5 but very slowly at pH 6.0. S1 nuclease could hydrolyze poly(rU) at pH 4.6, at a rate similar to that of ssDNA but poly(rC) was degraded at a slower rate (5%). Under similar conditions, poly(rA) and poly(rG) were resistant to hydrolysis. However, at pH 6.4, the enzyme could degrade poly(rC) and poly(rA) at a rate of 30% and 50%, respectively, to that of ssDNA [9]. Mung bean nuclease showed higher activity on poly(U) than poly(A) at pH 5.0 [172], and the susceptibility of the former was attributed to the lack of ordered secondary structure. Divalent cations influence the secondary structure of the polynucleotides. N. crassa (mycelia and conidia) nuclease showed specificity for polynucleotides lacking an ordered structure. Poly(dC) in the presence or absence of Mg2+ and poly(dI) in the absence of Mg2+ form random coil at pH 8.2 and were hydrolyzed by N. crassa nuclease at rates comparable to those of denatured DNA. However, alternating polymer poly(dIdC), which exists in the range of helix to coil transition in the absence of Mg2+ at 37°C, was hydrolyzed at a slower rate. On the other hand, poly(dAdT) and poly(dI) exist in the helical form in the presence of Mg2+ and were degraded at a rate similar to that of native DNA. Poly(dG) and poly(dGdC) which formed highly ordered structures at pH 8.2 were totally resistant to hydrolysis [112]. Wheat chloroplast nuclease hydrolyzed various synthetic polymeric substrates in the order poly(A)>poly(U)>poly(C)>poly(G)>poly(dA)>poly(dT)>poly(dC)>poly(dG) [35]. However, the nuclease from a fraction of wheat chloroplast stromal protein catalyzed the hydrolysis of polynucleotides in the order poly(U)>poly(A)>poly(C)>poly(G)>poly(dA)>poly(dT), whereas poly(dG) and poly(dC) were resistant to hydrolysis [36]. Nuclease I from rye germ ribosomes showed high specificity for poly(C) while the remaining ribopolynucleotides were hydrolyzed in the order poly(A)>poly(U)=poly(G). Although this enzyme hydrolyzed the double-stranded deoxyriboheteropolymer poly(dT)·poly(rA) at a very slow rate, it failed to hydrolyze the riboheteropolymer poly(A)·poly(U), suggesting its preference for single-stranded nucleic acids [100]. Similarly, the relative rates of hydrolysis of various synthetic polyribonucleotides by the acid and neutral nucleases from alfalfa seeds were in the order poly(U)>poly(A)>poly(C)>poly(G) and poly(A)≥poly(U)>poly(C)>poly(G), respectively, probably because the single-stranded character of the substrates decreased in the same order [37]. Sawai et al. [186] noted that the nuclease from carrot tissue cultures is the only plant nuclease that exhibits RNase H activity. Like rye germ ribosome nuclease [100], barley nuclease [94] hydrolyzed the polynucleotides in the order poly(C)>poly(U)>poly(A)>poly(A)·poly(U)>poly(G)=poly(G)·poly(C). A chromatin-bound deoxyribonuclease from the embryo axis of germinating pea exhibited maximum activity on the purine analogue polymer, poly(dI), followed by poly(dA), poly(dT) and poly(dC), suggesting its preference towards purines. The enzyme hydrolyzed the synthetic alternating copolymer poly(dA-dT):poly(dA-dT) 10-fold faster than the duplex copolymer poly(dG-dC):poly(dG-dC) [39]. This observation, coupled with the limited extent of hydrolysis of native DNA, suggested that the sites of action of the DNase in native DNA are the regions that exhibit ‘structural breathing’, i.e. transient single-stranded regions in DNA. Such regions are the AT-rich regions in DNA [156]. Similar observations were made in the case of mung bean nuclease [158]. In contrast, nuclease II bound to rye germ ribosomes hydrolyzed the double-stranded polymer poly(I)·poly(C) at a higher rate than poly(A)·poly(U), followed by the single-stranded polymers in the order poly(U)>poly(A)>poly(C)>poly(I)>poly(G) [187]. Nuclease Bh1 hydrolyzed various ribopolynucleotides in the order poly(A)≫poly(U)≫poly(A)·poly(U), while poly(G) and poly(C) were resistant to cleavage. The low susceptibility of the double-stranded polymer poly(A)·poly(U) suggested that nuclease Bh1 is a single-strand preferential enzyme [129].
10.2 Action on supercoiled and covalently closed DNA
Closed circular duplex DNA exists in a supercoiled form in plasmid and phage DNAs, which at sufficiently high negative superhelical density promotes unwinding of helical twists [188]. In the absence of strand breakage, the unwinding of one turn of the double helix allows the untwisting of one negative supercoil. The negative supercoiling of DNA in prokaryotes is essential for cell growth and is required to promote strand unwinding and separation which occur during DNA replication, transcription and recombination [189, 190]. As stated earlier, single-strand-specific nucleases have been shown to play an important role in DNA replication and recombination. Thus, it is likely that some sites in supercoiled DNA that exist transiently as single-stranded regions are susceptible to single-strand-specific nucleases. Almost all of the single-strand-specific nucleases reported so far have been shown to cleave supercoiled DNA from various sources [125]. These enzymes nick the supercoiled DNA (Form I) to give rise to relaxed circular DNA (Form II) and then to linear duplex DNA (Form III). However, the rate at which the Form II DNA is further converted to Form III DNA varies among the different nucleases. Thus S1 [191], mung bean [192] and N. crassa (mitochondria and vacuoles) nucleases [91] showed a high degree of specificity for Form I DNA and converted it rapidly to Form II DNA. In the case of mung bean nuclease, 28 000-fold excess enzyme was required to cleave the relaxed topoisomer (Form II) to Form III DNA, whereas with S1 nuclease very high concentrations were required for the conversion of Form II DNA to Form III DNA. The aforementioned enzymes cut each strand of DNA only once, i.e. they first nick superhelical DNA in one strand and then cleave the strand opposite the nick to generate unit length linear Form III DNA. It is interesting to note that snake-venom phosphodiesterase also exhibits single-strand-specific endonuclease activity with a similar preference (10 000-fold) for supercoiled over relaxed PM2 DNA. However, unlike mung bean nuclease, it does not accumulate the nicked circular DNA but cuts Form II DNA exactly opposite to the nick on the opposite strand. Thus, snake-venom phosphodiesterase can be used as an excellent tool for the specific cleavage of the strand opposite nicks containing 3′-OH and 5′-PO4 termini in duplex DNA [193]. Low concentrations of nuclease Bh1 converted Form I DNA to Form III DNA via the formation of Form II DNA. The enzyme could also linearize covalently-closed single-stranded M13 DNA, suggesting an endonucleolytic mode of action. Moreover, the inability of low concentrations of the enzyme to degrade Form III DNA, even on prolonged incubation, was correlated to the high single-strand specificity of the enzyme [20]. In the case of N. crassa nuclease, the enzyme action can be controlled by adjusting the concentration of Mg2+ ions in the reaction mixture. Low concentrations of the enzyme, in the presence of 0.1 mM Mg2+, exhibit strict endonuclease activity and high specificity for Form I DNA. However four- to eight-fold excess enzyme, in the presence of 10 mM Mg2+, accelerated the conversion of Form II DNA to Form III DNA and, subsequently, Form III DNA was degraded exonucleolytically [91]. In contrast, meiotic nuclease III from C. cinereus in the presence of 0.5 mM Mg2+ could not only nick supercoiled pBR322 DNA but also simultaneously produce the linear duplex DNA (Form III). Though with an increase in Mg2+ concentration (1 to 10 mM) increasing amounts of Form II and III DNA were observed, their ratio remained unchanged. However, at higher Mg2+ concentrations (>10 mM) the formation of Form III was gradually inhibited and a further increase in Mg2+ concentration showed a corresponding inhibition in the formation of Form II DNA. However, in presence of Ca2+ ions, not only was Form I DNA converted to Form III DNA, but a few products were also observed between Form I and Form III DNAs [33]. In the case of nuclease α from U. maydis, the ratio of the rate of hydrolysis of superhelical DNA to that of the relaxed DNA was highest (approximately 140-fold) when the reactions were carried out in the presence of 40–100 mM NaCl. In the absence of added salt, hydrolysis of Form II DNA proceeded at approximately one-tenth the rate of hydrolysis of Form I DNA [27]. The nicks generated by S1 [194], C. cinereus[31], mung bean [192] and Bh1 [20] nucleases and nuclease γ from U. maydis[29] were single base nicks, since they could be ligated by T4 DNA ligase to yield covalently closed circular DNA. In contrast, the nicks generated in supercoiled DNA by BAL 31 nuclease [195] and snake-venom phosphodiesterase [193] could not be ligated back to covalently closed DNA, since they were extended into gaps by the exonuclease action of these enzymes.
Certain inverted repeats in supercoiled DNA adopt a hairpin or cruciform configuration [196, 197]. Asakura et al. [198] demonstrated that such inverted repeats are found in yeast 2μ DNA and S1 nuclease cleaves at the center of the palindrome 3 which adopts a cruciform structure. Panayotatos and Wells [199] showed that such cruciform structures also occur in pBR322 and pVH51 plasmid DNAs and that they exhibit a similar cleavage pattern on treatment with endonucleases such as S1 and the T7 gene 3 product.
It is known that intercalating agents change the superhelical density of plasmid DNA in the order: less negatively supercoiled→relaxed→positively supercoiled. Moreover, negatively supercoiled DNA is known to form stably unwound DNA conformations, including Z-DNA, cruciform and homopurine–homopyrimidine structures. BAL 31 nuclease cleaves very highly supercoiled DNA prepared from covalently closed relaxed DNA (Form I°) with ethidium bromide [96]. Initial nicking rates of PM2 Form I DNA by BAL 31 nuclease are readily measurable at superhelical densities as low as −0.02, while the initial nicking with nucleases from N. crassa and mung bean requires more negative superhelicity. Nicking of positively supercoiled DNA by BAL 31 nuclease becomes detectable at superhelical densities between 0.15 and 0.19 [9].
An endonuclease from Salmonella typhimurium was shown to cleave both positively and negatively supercoiled DNA. However, when the supercoiled DNA was converted to a fully relaxed form with the help of ethidium bromide, no further conversion of this relaxed DNA was observed [200]. Similarly, meiotic nuclease I from C. cinereus could not hydrolyze the relaxed PM2 DNA generated by the action of topoisomerase I [31]. C. fasciculata nicking enzyme cleaves a single phosphodiester bond in duplex DNA circles only in their supercoiled form, but not following their relaxation by topoisomerases. However, the requirement of DNA supercoiling was not observed with kinetoplast DNA [52]. The capacity of the enzyme to activate a relaxed DNA topoisomer for nicking is an intrinsic property of the sequence-directed bend naturally present in kinetoplast DNA. The 211-bp fragment of the bent region of C. fasciculata kinetoplast DNA served as the unique binding site for the nuclease. Sequence analysis of the nicking sites in both strands of the 211-bp bent fragment revealed the presence of 32 nicking sites within the sequence. Twenty-six of the 32 nicking sites were located within the dinucleotide sequences ApA (10), TpT (10), ApT (5) and TpA (1) in both strands. This observation indicated that the preferred site of cleavage lies in an A+T-rich region [201]. Similar observations were made with mung bean nuclease [158]. Nuclease hypersensitivity of a variety of supercoiled DNAs occurs extensively in specific A+T-rich sequences and is influenced by temperature and ionic strength [202–205], and this hypersensitivity can shift to sequences that form cruciforms or Z-DNA under different conditions [206]. Kowalski et al. [207] demonstrated that the stable DNA unwinding, as opposed to transient unwinding or breathing, is the reason for single-strand-specific nuclease hypersensitivity of specific A+T-rich regions.
The DNA polymer (dC-dG)n·(dC-dG)n exists in a left-handed conformation in the presence of high salt concentrations [208]. Furthermore, segments of (dC-dG) in DNA restriction fragments and in recombinant plasmids adopt a left-handed conformation in high salt solutions, while the neighboring regions of natural sequences remain in right-handed helices. S1 nuclease specifically recognizes and cleaves the junction between right- and left-handed regions [209–211]. However, its use as a B–Z junction probe is restricted to supercoiled DNA due to its inhibition at high salt concentrations. Kilpatrick et al. [212] showed that BAL 31 nuclease cleaves the B–Z junction in high salt concentrations, but it does not cleave DNA under conditions where (dC-dG)n blocks exist in the B conformation. For these S1 nuclease is a more specific probe, since it lacks the exonucleolytic activity. In contrast, the DNA/RNA non-specific Serratia[213] and Anabaena[169] nucleases prefer the double-stranded A-form of the nucleic acids.
Endonucleases are also capable of cleaving covalently closed circular DNA. Thus, S1, mung bean, F. velutipes and U. maydisα, β and γ nucleases and nuclease Bh1 hydrolyzed covalently closed circular ssDNA. Meiotic nuclease II from C. cinereus[144], though giving endonucleolytic fragmentation of circular M13 ssDNA, did not result in the formation of acid soluble products, since the 3′-PO4 termini generated were refractory to further processing by the exonuclease activity of the enzyme. While the nature of the digestion products and the mode of degradation of linear DNA suggested an endonucleolytic action, the single-strand-specific nuclease from U. maydis failed to cleave circular φX174 ssDNA and polyoma Form I and Form II DNAs. However, the resistant circular DNAs could be made susceptible by converting them into linear form. Hence the authors opined that the nuclease probably requires DNA with free ends for its activity [142].
10.3 Action on oligonucleotides that give rise to single-stranded loops
Duplex DNA molecules with covalently closed (hairpin) ends are formed as a result of several biological processes, such as replication of some phage and viruses [214, 215], site-specific recombination [216, 217], and retroviral integration and transposition [218]. Moreover, hairpins have also been implicated as intermediates in the excision of transposable elements in plants [219] and in the rearrangement of T-cell receptor and V(D)J recombination [220, 221]. Kabotyanski et al. [222] demonstrated the use of P1 and mung bean nucleases in opening the hairpin ends and concluded that the action of these enzymes is dependent on the sequence of the oligonucleotides that form the hairpins. Thus, P1 nuclease preferentially cleaves the 3′ of A residues [9], whereas mung bean nuclease cleaves the 3′ of A and T residues in ssDNA [158]. Interestingly, these enzymes do not efficiently remove the short single-stranded tails generated from the hairpins following the initial cleavage. The inability of S1 nuclease to remove short single-stranded extensions [9] is in accordance with the observations of Kabotyanski et al. [222]. Mung bean nuclease showed a strong preference for opening the hairpin bends at pH 7.4 compared to the single-stranded 21-mer substrate. Even at very high enzyme concentrations, the single-stranded 21-mer polynucleotide was highly resistant to cleavage. However, at pH 5.3 both the substrates were highly susceptible to the enzyme action [222]. Similar observations were made with P1 nuclease. Based on these observations, the authors [79] opined that hairpin opening by P1 and mung bean nucleases is remarkably efficient compared to the degradation of ssDNA. Moreover, in the case of mung bean nuclease the cleavage in the loop region was dependent on the stacking of the bases adjoining the loop. In the presence of high concentrations of Mg2+, the double helical loop is stabilized and hence there is restricted access of the enzyme to the loop bases adjacent to the helical regions. This was correlated with reduced exposure of the bases to nuclease action in the presence of high concentrations of Mg2+[79]. S1 nuclease, owing to a similar ability to cleave loops, has been used as a probe for the anticodon loop of tRNA [223, 224]. Drew [10] used oligonucleotide substrates that form double strands as well as hairpin loops and demonstrated the ability of five nucleases to distinguish several different DNA backbone configurations (Fig. 1). DNase I can cut only one strand at a time and so it should turn over into a second orientation to cleave the opposite strand (Fig. 1a). Nevertheless, it appears that DNase I prefers a double-stranded phosphate track of suitable gauge as a binding site. Where the groove widens into a loop or where a well-defined groove ceases to exist near the 5′-end, the cleavage rate of the enzyme decreases dramatically. S1 nuclease and micrococcal nuclease (Fig. 1b,c) are very similar in their specificities. The difference between these two enzymes is that S1 nuclease does not show any base preference in the single-stranded regions, in the loop or at the ends of the hairpin, whereas micrococcal nuclease cuts preferably in the AT-rich regions. Copper/phenanthroline (a chemical nuclease; Fig. 1d) prefers a duplex to a loop, probably because it intercalates between base pairs. DNase II (Fig. 1e) does not require a double-stranded track like DNase I, nor does it require an exposed phosphate group like S1 and micrococcal nuclease. It has a narrow binding domain and can reach down into the groove of the double helix so as to bind one strand with minimal interference from the opposing strand. However, the above studies indicated that the binding sites of the nuclease on the substrate and the site of cleavage might vary. For example, micrococcal nuclease binds two phosphate residues to the 3′-side of where it cuts [225]. S1 nuclease, on the other hand, cuts symmetrically about the tip of the loops and so its cutting site is near its binding site. Similarly, Lilley [226] showed that S1 nuclease cuts oligomer hairpins and duplexes near 5′- and 3′-ends in unpaired loops. It was also noted that when two hairpins oppose each other to form a cruciform structure, the unpaired loops become more sensitive to S1 nuclease than any other structural feature(s). It appears that the accessibility of S1 nuclease to the substrate is substantially restricted in the central region of the cruciform structure as compared to the exposed and protruding loops. Moreover, using a small single-strand-specific reagent (bromoacetaldehyde) to probe the unpaired bases, Lilley [226] showed that the region of single-strand near the base of the cruciform structure, 10–15 bp on either side of the loop tip, becomes more reactive than the loop itself. This observation was consistent with the notion that S1 nuclease requires a greater degree of phosphate exposure than other single-strand-binding proteins such as micrococcal nuclease and DNase II. Using oligonucleotide substrates containing a run of five dG bases and substitution of the dG bases with deoxyguanosine analogues, Cal et al. [227] showed that S. antibioticus nuclease interacts with both strands of DNA and also contacts the nucleic acid in both the major and minor grooves.
DNA configurations recognized by five different nucleases [B, bond attacked; S, structure attacked; P, preferred conformation]. a: DNAase I: B: O-3′-P; S: two sugar–phosphate strands that are closely spaced; P: minor groove of duplex. b: S1 nuclease: B: O-3′-P; S: exposed single-strand; P: ends of duplex, tip of loop. c: Microccal nuclease: B: O-5′-P; S: exposed single-strand, unpaired A or T base. P: 5′-end of duplex, side of loop, weakly in duplex. d: Copper–phenanthroline: B: sugar ring; S: basepair step; P: duplex. e: DNase II: B: O-5′-P; S: slightly exposed, stacked single-strand; P: duplex with wide minor groove, stacked loop. Reprinted with permission from Drew [10].
10.4 Base/linkage specificity
Although the majority of single-strand-specific nucleases reported so far are base non-specific, the enzymes from N. crassa, U. maydis, P. citrinum, B. haptosporus, mung bean, avena leaf and spinach show some base specificity during the initial stages of hydrolysis. An endo-exonuclease from N. crassa (conidia) [112], U. maydis[228], B. haptosporus[20] and nuclease C from Chlamydomonas reinhardtii[229] showed a preference for guanylic acid linkages in ssDNA. However, S. antibioticus nuclease [227] showed a preference for runs of dG bases in dsDNA but not in ssDNA. Double-stranded oligonucleotides containing sequences of four or more consecutive deoxyguanosine residues were preferentially hydrolyzed, with the strongest cutting site occurring at GGG↓GG. Moreover, 5′-terminal analysis of the cleavage products of dsDNA by S. antibioticus nuclease [227] as well as that of a site-specific single-strand-specific endonuclease from Chlamydomonas sp. [230] showed the predominance of dG followed by dT, suggesting that after d(GpG) linkages d(GpT) linkages are preferred. Several nucleases are known which are not strictly single-strand-specific but show a preference for runs of dG bases. These include the extracellular nuclease from S. marcescens[169] and endonuclease G from mammalian nuclei and mitochondria [231–234]. However, the other properties of these enzymes do not match those of other single-strand-specific nucleases. Thus, S. marcescens nuclease cuts runs of dG bases in ds DNA but not in d(A)·d(T) tracts, whereas in ssDNA it readily cleaves the d(A)·d(T) tracts. Although endonuclease G recognizes dG/dC tracts, both the purine and pyrimidine strands are cleaved with equal efficiency. However, in the case of guanylic acid preferential nucleases from N. crassa[112] and C. reinhardtii[229] cytidylic acid linkages were resistant to cleavage. In contrast, S. glaucescens nuclease recognized the dinucleotide sequence 5′-CC-3′ in dsDNA and cleaved 3′ to the first C and also demonstrated a marked preference for certain 5′-CC-3′. At a higher enzyme concentration (>10-fold), the sequences 5′-GC-3′, 5′-CG-3′ and 5′-GG-3′ were cleaved along with other 5′-CC-3′ sequences that were previously resistant. Similarly, a site-specific single-strand endonuclease activity induced by an NYs-1 virus infection in a Chlorella-like green alga recognized the two-base sequence 5′-CC-3′ and cleaved 5′ to the first C. In contrast to S. glaucescens nuclease, the Chlorella enzyme exhibited an absolute specificity for the dinucleotide sequence CpC in dsDNA and was stimulated by ATP. It cleaved 5′-CmC-3′ (where mC is methylcytosine) sequences but not 5′-mCC-3′ sequences. The enzyme generated breaks in dsDNA whenever two 5′-CC-3′ sequences on opposite strands were close enough for the two strands to separate. However, when the 5′-CC-3′ sequences on opposite strands were further apart, only a portion of the strands separated following enzyme action. Moreover, it did not act on single-stranded nucleic acids [235]. Single-strand-specific P1 [236], spinach [78] and avena leaf [41] nucleases preferentially attack adenylic acid linkages in DNA. Moreover P1 nuclease preferred 3′-ribonucleotide monophosphates to 3′-deoxyribonucleotide monophosphates [185]. Using a combination of 16 dinucleoside monophosphates as substrates, Box et al. [237] demonstrated that P1 nuclease shows maximum specificity for dinucleoside monophosphates having adenosine at the 5′-end and cytidine at the 3′-end. Thus, dinucleoside monophosphates having adenosine either at the 5′- or the 3′-end were hydrolyzed in the order d(ApC)>d(CpA)>d(ApG)>d(ApT)>d(ApA)>d(GpA)>d(TpA). In addition, dinucleoside monophosphates of the type d(TpN) were highly resistant to cleavage followed by d(GpN). Furthermore, dinucleoside monophosphates, having either deoxycytidine or deoxyadenosine as the 5′-nucleoside, were most susceptible to hydrolysis. Based on these observations, the authors [237] concluded that P1 nuclease prefers the AC linkages in DNA. These results also indicated that in a polymeric substrate, the bases adjacent to the most preferred base also influence the specificity of the enzyme. A study of the rates of hydrolysis of dinucleoside monophosphates by nuclease I from Nicotiana tabacum[117] revealed a strong preference for purine nucleosides as the 5′ residue, with slight preference for uridine as the 3′ residue. Similar observations were made with barley nuclease [94]. The actions of the acid and neutral nucleases from alfalfa seeds on dinucleoside monophosphates and deoxydecanucleotides suggested that they differ in their preference for the phosphodiester linkages. Acid nuclease hydrolyzed all dinucleoside monophosphates except UpU, CpC, CpA and CpG to their respective 3′-mononucleotides and nucleosides, while the neutral nuclease cleaved all dinucleoside monophosphates with the exception of UpU, UpC, UpG, CpA and CpG to their corresponding 5′-mononucleotides and nucleosides. Moreover, the action of these enzymes on deoxydecanucleotides showed that the acid nuclease exhibited a preference for various linkages in the order Cp↓C>Tp↓C>Gp↓T>Tp↓T>Ap↓C>Cp↓G>Gp↓G, whereas the neutral nuclease hydrolyzed various bonds in the order T↓pG>G↓pG>G↓pA>T↓pC>G↓pT>C↓pG>A↓pC [37]. In contrast, the nuclease from germinated lentil exhibited a preference for T↓pT>T↓pG>G↓pT>G↓pC>A↓pG>G↓pG linkages [238]. However, recent studies on the action of acid and neutral nucleases from alfalfa seeds on a synthetic 36-mer deoxynucleotide revealed that the acid nuclease showed an initial preference for Cp↓C>Cp↓A>Gp↓T>Gp↓C bonds while the neutral nuclease for Ap↓T>Cp↓T=Tp↓C bonds. The probable reason for the observed differences in the bond preference of these two enzymes in the 10-mer and 36-mer substrates, is that the specificity of a nuclease depends not only on the neighboring bases but also on the size of the substrates [118]. The action of nuclease Bh1 on deoxyribodinucleoside monophosphates showed that they are hydrolyzed in the order d(GpG)>d(TpT)>d(ApA). While d(GpG) was hydrolyzed completely, d(CpC) was resistant to cleavage. Moreover, dinucleoside monophosphates with deoxyguanosine at the 5′-end were cleaved more efficiently than ones with thymidine or deoxyadenosine as the 5′-nucleoside. Furthermore, dinucleoside monophosphates having the preferred base at the 5′-end were hydrolyzed to a greater extent than when it was present at the 3′-end. On the contrary, deoxyribodinucleoside monophosphates with deoxycytidine either at the 3′- or the 5′-end were resistant to hydrolysis [20].
Three site-specific nucleases have been isolated from S. cerevisiae. An endonuclease, designated YZ endo, cleaved at sites corresponding to the in vivo double-stranded breaks occurring at the mating-type interconversion [239]. YZ endo generates a site-specific double-stranded break having four base-3′ extensions terminating in 3′-hydroxyl groups. The cleavage occurs in the Z1 region near the YZ junction of the mating-type locus. A second endonuclease, SceII, was present in all the strains of S. cerevisiae examined by Kostricken et al. [240]. The cleavage site of SceII is unrelated to the YZ endo cleavage site. A third nuclease, Endo–SceI, introduces a double-stranded break in a 26-bp consensus sequence [241]. The endonuclease from Chlamydomonas sp. showed specificity for certain sites in adenovirus-2 DNA and the initial cleavage occurred at a site containing deoxythymidine residues. Hence, the authors [230] proposed that, after an initial endonucleolytic cleavage at a preferred site on one strand of duplex DNA, the enzyme moves along the DNA molecule displacing the DNA strand until it reaches another recognition site, where a second single-strand cleavage occurs, producing a gap in the duplex DNA and an excised oligonucleotide. Holdsworth et al. [242] characterized a novel site-specific endonuclease from C. fasciculata, Endo-A, for which the preferred site of cleavage was within the TpC dinucleotide of the sequence ANATC on one strand of the double-stranded DNA probe, where N is any nucleotide.
10.5 Action on modified substrates
Single-strand-specific nucleases, in addition to hydrolyzing natural substrates like DNA and RNA, act on alkylated, depurinated and UV-irradiated substrates and DNAs having single-base lesions, mismatched bases and heteroduplexes. N. crassa endonuclease cleaved UV-irradiated DNA in the region containing pyrimidine dimers [243]. The action of S1 nuclease on UV-irradiated DNA revealed that the cleavage consisted of both single and double-stranded breaks [244]. Double-stranded DNA breaks were also observed following the hydrolysis of γ-irradiated DNA [245, 246]. The above studies suggested that S1 nuclease recognizes alterations in the double helical structure produced by UV-irradiation rather than specifically attack the UV-induced photoproducts. Moreover, its action on UV-irradiated DNA was dependent on enzyme concentration, ionic strength of the reaction mixture and was directly proportional to the dose of UV-light. Hence, S1 nuclease has been used to monitor chemically induced disruption of DNA secondary structure [244]. The enzyme also acts on DNA treated with alkylating agents [247, 248], N-acetoxy-N-2-acetylaminofluorene [249] and cisplatin [250]. SP nuclease from spinach nicks UV-irradiated duplex DNA at adenine residues in the vicinity of 2–7 nucleotides on the same strand in response to the formation of TC(6–4) photoproducts but not cyclobutane-type pyrimidine dimers. DNA damaged with cis-diaminedichloroplatinum and N-acetoxy-N-acetyl-2-aminofluorene was also cleaved at adenine by SP nuclease. This suggested that the enzyme does not cleave DNA in response to specific adenine modification but rather incises DNA at adenine residues in the vicinity of helical distortions produced by photoproducts, platinum and other modifications. Hence, it was concluded that SP nuclease might be involved in the repair of the dimers formed due to UV-irradiation [42]. Enzymatic hydrolysis of the intradimer phosphodiester bond may constitute the initial step in the repair of UV-induced cyclobutane pyrimidine dimers in human cells. UV-irradiation of the trinucleotide, d-TpTpT, results in the formation of two isomeric compounds containing a cis-syn-cyclobutane dimer. Action of various nucleases on these isomers showed that snake-venom phosphodiesterase hydrolyzed only the 3′-phosphodiester group in the 5′-isomer (d-T<p>TpT) but was totally inactive toward the 3′-isomer (d-TpT<p>T). In contrast, calf spleen phosphodiesterase acted only on the 3′-isomer by cleaving the 5′-internucleotide bond. Kinetic analysis revealed that: (i) the activity of snake-venom phosphodiesterase was unaffected by a dimer 5′ to the phosphodiester linkage, (ii) the action of calf-spleen phosphodiesterase was partially inhibited by a dimer 3′ to the phosphodiester bond, and (iii) E. coli phrB-encoded DNA photolyase reacted twice as fast on d-T<p>TpT than d-TpT<p>T. Mung bean, S1 and P1 nucleases cleaved the 5′-internucleotide linkage but not the intradimer phosphodiester bond in d-TpT<p>T. Similarly, both phosphate groups in d-T<p>TpT were resistant to mung bean and S1 nuclease. Interestingly, incubation of d-T<p>TpT with P1 nuclease, however, generated the novel compound d-T<>d-pTpT containing a severed intradimer phosphodiester linkage. Thus, P1 nuclease represents the first single-strand-specific enzyme known to hydrolyze an intradimer phosphodiester linkage [251].
Oleykowski et al. [252] showed that a mismatched DNA duplex is also an excellent substrate for SP nuclease. In base substitution mismatches, SP nuclease incises at all mismatches except those containing a guanine residue. It also cleaves at insertions/deletions of one or more nucleotides. In the case of an extrahelical loop containing one nucleotide, the preference for cleavage of nucleotides was A≫T∼C but not G. In contrast, the CEL I family of nucleases from the leaves of broccoli, cabbage, cauliflower and celery could incise substrates with extrahelical loops containing all four nucleotides. This property of nuclease CEL I was used for the detection of mutations and polymorphisms of the BRCA1 gene in a number of women affected with either breast and/or ovarian cancer and reporting a family history of these ailments. The principle of mismatch recognition by CEL I appears to be different from T4 endonuclease VII, which is actually a resolvase that nicks one strand at the site of a mismatch and then in the strand opposite the nick [253]. However, CEL I nuclease nicks only one strand of DNA in a mismatch heteroduplex at the site of the mismatch. In contrast, heteroduplex DNAs containing single base mismatches are highly resistant to S1, P1 and mung bean nucleases [254]. Under conditions where little or no non-specific DNA degradation was observed, all three nucleases could generate double-stranded breaks when the bistranded abasic sites were one to three bases apart. Structural studies indicated that the disruption caused by the introduction of an abasic site in duplex DNA extends to the adjacent base pairs [255, 256]. With the abasic sites 1–3 bp apart, destabilization of the duplex would effectively extend over 4–6 bp, making the DNA susceptible to the nucleases. However, when these abasic sites were further apart, they were not cleaved by S1, P1 and mung bean nucleases since these sites were effectively single abasic sites [254]. A single apurinic site was sufficient to elicit BAL 31 cleavage of duplex DNA wherein the F form was 2.5-fold more efficient in cleaving the depurinated DNA than the S species [257]. Nuclease α from U. maydis cleaved depurinated, deaminated and UV-irradiated DNA at considerably higher rates than the untreated DNA. Heteroduplexes containing single mismatched base pairs were cleaved at the same rate as those of the normal substrates in presence of 10 mM Mg2+ and 50 mM NaCl. However, when the reaction conditions were changed by replacing Mg2+ and NaCl with 0.2 mM Co2+ and lowering the temperature from 37 to 20°C, the heteroduplex DNA was cleaved somewhat faster than the control DNA. The higher activity of the enzyme under changed experimental conditions was correlated to two kinds of structural changes in DNA [27]. First, some negative superhelical turns may have been introduced by carrying out the reaction at a temperature 17°C lower than the ligation reaction [258, 259]. Secondly, at the concentration employed, Co2+ might destabilize the DNA helix and cause some tilting of bases [260, 261]. These structure-distorting treatments coupled with the known destabilizing effect of the mismatch may render the heteroduplex DNA more susceptible to nuclease α than the homoduplex DNA [27]. Similarly, Naseem and Hadi [88] showed that pea seed nuclease hydrolyzed alkylated and depurinated DNA at rates higher than that of native DNA. Moreover, the enzyme preferentially hydrolyzed depurinated DNA at apurinic sites rather than apyrimidinic sites. Weinfeld et al. [262, 263] studied the hydrolysis of phosphodiester bonds adjacent to apurinic sites by S1, P1 and mung bean nucleases, using dinucleotides lacking either a 5′- or a 3′-base as substrates. Partial depurination of d-ApA produced two A260 absorbing isomers, d-SpA and d-ApS (where S represents the depurinated deoxyribose sugar). S1, P1 and mung bean nucleases hydrolyzed the d-ApS isomer but not the d-SpA isomer, indicating that they interact with the base 5′ to the internucleotide phosphate group. Stacking between aromatic amino acids of nucleases and nucleic acid bases plays an important role in the interaction of single-strand-specific nucleases with their substrates. Moreover, Weinfeld et al. [264] studied the requirements of DNA base aromaticity for five enzymes (T4 polynucleotide kinase, P1 and S1 nucleases, and snake-venom and calf-spleen phosphodiesterases) acting on ssDNA. The modified substrates contained either cis-5R, 6S-dihydro-5, 6-dihydroxythymidine (thymidine glycol) or a mixture of 5R and 5S isomers of 5,6-dihydrothymidine. It was observed that for all the enzymes except snake-venom phosphodiesterase the parent molecules were better substrates than the dihydrothymidine derivatives, while the thymidine glycol compounds were very poor substrates. Snake-venom phosphodiesterase acted on the unmodified and dihydrothymidine molecules at almost the same rate. P1 and S1 nucleases hydrolyzed the molecules containing 5R-dihydrothymidine approximately fifty times faster than those containing the S-isomer. The other enzymes displayed no measurable stereospecificity. Potter et al. [265] used S1 nuclease, for determining the stereochemical course of hydrolysis of DNA, to assess the presence or absence of a covalent enzyme intermediate in reactions catalyzed by single-strand-specific nucleases. The action of S1 nuclease on distereoisomers of d[Tp(S)A] indicated that the hydrolytic reaction proceeds with the inversion of configuration of the oxygen atom on the phosphorous. Similar results were obtained with P1 nuclease [266], indicating that the hydrolytic reaction does not involve the formation of a covalent enzyme substrate intermediate.
10.6 Structure-specific DNA cleavage
Several important pathways of DNA metabolism involve the transient formation of branched DNA structures that contain free 5′-single-stranded ends. Such structures can arise during replication, recombination and repair. Processes such as nick translation or removal of Okazaki fragments from the lagging strand during DNA replication results in the formation of a displaced strand or a bifurcation, termed a ‘flap’. These flap structures are cleaved by structure-specific 5′→3′-exonucleases, many of which are physically associated with DNA polymerases (Fig. 2a,b).
A: Substrates of 5′→3′-exonucleases. B: Action of 5′-nuclease on flap structure during nick translation. The invading primer is shown in bold line. It is extended by the action of Pol I and the displaced strand forms a flap structure. The endonucleolytic cleavage by 5′-nuclease is indicated by a bold arrow, whereas the faded arrow indicates exonucleolytic attack. (Reprinted from Ceska and Sayers, [267], ©1998, with permission from Elsevier Science.)
Two well-studied enzymes of bacteriophage origin, the λ exonuclease and T5 D15 5′→3′-exonuclease, hydrolyze DNA in a 5′→3′ direction and require a divalent cation for activity [267]. The λ exonuclease releases only mononucleotides from the 5′-end of duplex DNA, provided that a 5′-terminal phosphate group is present, but it cannot hydrolyze ssDNA or RNA. It does not act on substrates that have 5′-single-stranded overhangs and show no endonuclease activity [268]. In contrast, T5 exonuclease digests dsDNA and ssDNA, releasing mono-, di- and tri-nucleotides as well as oligonucleotides and can also act on DNA–RNA hybrids [269]. A mammalian flap endonuclease (FEN-1), characterized by Harrington and Lieber [270] and previously identified as DNase IV by Lindahl et al. [271], exhibits properties that are very similar to those of prokaryotic 5′-nucleases. Lyamichev et al. [272] proposed that this enzyme gains access to the cleavage site by moving from the free end of a 5′ extension to the bifurcation of the duplex, where the endonucleolytic cleavage takes place. Single-stranded 5′ arms up to 200 nucleotides long are cleaved from such a duplex.
11 Associated phosphomonoesterase activity
Most of the extracellular single-strand-specific nucleases exhibit an associated phosphomonoesterase activity. The main role of nucleotidases is to scavenge nucleosides and phosphates for growth which, in turn, is useful for the survival of the organism under environmentally stressed conditions. This is supported by the observation that high levels of 3′-phosphomonoesterase activity were induced in trypanosomes in response to depletion of purines and inorganic phosphate in the growth medium [135]. S1 [128], P1 [185], mung bean [273], pea seed [88] and potato tuber [171] nucleases possess 3′-phosphomonoesterase activity. However, nuclease β from U. maydis dephosphorylates 5′-mononucleotides [28]. The 3′-nucleotidase activity of all the single-strand-specific nucleases reported to date shows a strong preference for ribonucleotides over deoxyribonucleotides. In fact, 3′-deoxyribomononucleotides were resistant to the 3′-nucleotidase-nuclease from C. luciliae[135] and nuclease Bh1 [20]. Thus, mung bean [273], P1 [185] and tobacco [117] nucleases cleave 3′-ribonucleotides 20–50-fold faster than the 3′-deoxyribonucleotides. The preference for ribonucleotides was correlated to the presence of the 2′-OH group in the ribonucleotides [273]. In addition, some nucleases exhibit a base preference for the hydrolysis of various 3′-mononucleotides. Thus, P1 nuclease hydrolyzed various 3′-ribonucleotides in the order G>A>C≥U, whereas the 3′-deoxyribomononucleotides were hydrolyzed in the order C≥T>A≥G. P1 nuclease also acts on nucleoside 3′→5′-diphosphates but 2′-AMP is highly resistant to cleavage [185]. The substrate specificity of S1 nuclease is in the order ribonucleotide 3′,5′-diphosphate>ribonucleotide 3′-phosphate>Deoxyribonucleotide 3′,5′-diphosphate>deoxyribonucleotide 3′-phosphate∼ribonucleotide 2′-phosphate [128]. The base specificity of tobacco nuclease is in the order A>G≫U>C and a greater affinity was observed when the substrate contained an additional phosphate at the 5′ position [117]. Similar observations were made in the case of S1 [128] and mung bean [273] nucleases. Nuclease Bh1 dephosphorylated 3′-ribonucleotides in the order 3′UMP>3′AMP≫3′GMP>3′CMP. Moreover, the presence of an additional phosphate at the 5′ position did not influence the cleavage efficiency [130].
12 Structure and function
The N-terminal amino acid sequences of six single-strand-specific nucleases have been deduced and all of them have tryptophan as the N-terminal residue (Fig. 3). The C-terminal amino acid residues of S1 [119], P1 [274] and PA3 nucleases [275] are serine, leucine and isoleucine, respectively. The amino acid sequence of a nuclease from Penicillium sp. was found to be identical to that of P1 nuclease except that Thr 190 was replaced by Ile in P1 nuclease [275]. The complete amino acid sequences of the S1 and P1 nucleases have been determined and they showed approximately 50% similarity [119]. However, the N-terminal amino acid sequence of the intracellular nuclease O from A. oryzae showed no sequence similarity with the extracellular S1 nuclease [276]. Similarly, the N-terminal sequence of meiotic endonuclease I from C. cinereus did not show any similarity with the above nucleases [277].
Comparison of N-terminal amino acid sequence of single-strand-specific nucleases.
Although a large number of single-strand-specific nucleases have been reported to date, structural studies have been limited to the S1 and P1 nucleases. S1 nuclease consists of a single polypeptide chain of 267 amino acids cross-linked by two disulfide bonds. The disulfide (S–S) linkages probably occur with Cys 72 to Cys 216 and Cys 80 to Cys 85. It is a high mannose-containing glycoprotein and the sugar moieties are attached to asparagine 92 and 228 [119]. P1 nuclease also consists of a single polypeptide chain made up of 270 amino acids with two disulfide bonds, Cys 72 to Cys 217 and Cys 80 to Cys 85. It contains four carbohydrate moieties linked to asparagine 92, 138, 184 and 197. Based on circular dichroism (CD) and optical rotatory dispersion studies, Fujimoto et al. [278] showed that P1 nuclease consists of 29–31%α-helix, 6%β-sheet and 63% random coil, whereas S1 nuclease consists of 25%α-helix, 31%β-sheet and 44% random coil [132]. Recently, S1 nuclease has been cloned and the full gene sequence deduced [279]. Also the nuclease O gene of the intracellular nuclease from A. oryzae was isolated and characterized [276]. Similarly, meiotic endonuclease I from C. cinereus was also cloned by Charlton et al. [277]. Comparison of the gene sequences of S1 nuclease and nuclease O from A. oryzae did not reveal any sequence similarity. P1 nuclease was crystallized in three different space groups and its structure was studied at 4.5 Å resolution [280]. Subsequently, Volbeda et al. [281] solved its structure at 3.3 Å and refined the data at 2.8 Å. The three-dimensional structure of P1 nuclease showed the presence of 269 amino acid residues, three zinc atoms and two N-acetylglucosamine residues. The C-terminal lysine 270 could not be located in the electron density map. The main chain folding of the enzyme was very similar to that of phospholipase C from Bacillus cereus, with 56% of the structure exhibiting α-helical conformation. This value, however, shows a considerable variation from the one obtained from CD data.
12.1 Coordination and function of metal ions
P1 nuclease is a zinc metalloprotein and contains three Zn2+ atoms per molecule of the enzyme. Rokugawa et al. [282] carried out a systematic investigation on the role of metal ions in P1 nuclease by selective removal of zinc from the enzyme by EDTA treatment and noted that the activity loss towards RNA and 3′-AMP is related to the removal of the number of zinc atoms. The removal of one zinc atom from the enzyme resulted in a 50% loss of its activity towards RNA but it retained 93% activity towards 3′-AMP. While the removal of second zinc atom brought about further decrease in the RNase activity (45%) and a significant decrease in the phosphomonoesterase activity (60%), the removal of all the three zinc atoms resulted in complete inactivation of the enzyme and a complete disruption of the enzyme structure. Based on these observations the authors concluded that, while zinc I is involved in maintaining the tertiary structure required for RNA binding, zinc II is essential for maintaining the active conformation and zinc III is involved in structural integrity of the enzyme. Like P1 nuclease, S1 nuclease is also a zinc metalloprotein and contains three atoms of zinc per molecule of enzyme. Shishido and Habuka [132] demonstrated that the removal of two zinc atoms from the enzyme results in the irreversible inactivation of the enzyme and the inactivation is due to the disruption of its secondary structure. Subsequently, Gite and Shankar [283] showed the involvement of carboxylate groups in metal binding. The data obtained with carboxylate-group modification, EDTA treatment, reconstitution with metal ions, zinc estimation and CD analysis of the enzyme suggested that, of the three zinc atoms present in S1 nuclease, zinc I is easily replaceable and is probably involved in catalysis, whereas zinc II and III are involved in maintaining the enzyme structure.
The crystal structure of P1 nuclease revealed that the zinc cluster is located at the bottom of the substrate binding cleft. It consists of a relatively inaccessible dinuclear site with two zinc ions separated by 3.2 Å and a more exposed single site roughly 5 Å away from the other two zinc ions. Both zinc ions of the dinuclear pair are coordinated to four protein ligands and a bridging water molecule, while the more exposed zinc ion (ZnII) is linked to only three amino acid ligands (His126, His149 and Asp53) and two water molecules (Fig. 4). The coordination of ZnII resembles those seen in all structurally characterized catalytically active zinc sites reported so far, Valle and Auld [284] suggesting that it is directly involved in catalysis. The dinuclear zinc pair probably has an important function in stabilizing the fold of P1 nuclease. It tightly links together three regions which are far apart in the amino acid sequence [285].
Coordination of zinc ions in P1 nuclease. 01, 02, 03 represent the water ligands. (Reprinted from Volbeda et al. [281], ©1991, with permission from Oxford University Press.)
The endonuclease from S. marcescens is a Mg2+-dependent DNA/RNA non-specific nuclease, whereas Staphylococcal nuclease is a Ca2+-requiring enzyme and neither enzyme exhibits any preference for single-stranded nucleic acids. Although the discussion of all the properties of these non-specific nucleases is outside the scope of this review, they are noteworthy, since extensive studies on these enzymes have provided insights into the substrate binding and catalytic mechanism. In the Serratia nuclease, the Mg2+ atom is coordinated with Asn119 and five solvent molecules arranged in an octahedron around the central metal atom. His89 and Glu127 are in close proximity to Mg2+ but do not directly interact with the metal ion [286]. On the other hand, in Staphylococcal nuclease, Ca2+ is coordinated to Asp40 and Glu43 and a water molecule [287].
12.2 Substrate binding and catalysis
Although considerable work has been done on the substrate specificity and mode of action, very little information is available regarding the active site nature of single-strand-specific nucleases. Through competitive inhibition studies, it was demonstrated that the ssDNase, RNase and phosphomonoesterase activities associated with S1 nuclease [128], P1 nuclease [288] and nuclease Bh1 [130] are catalyzed by the same active site. Reddy and Shankar [289], while studying the immobilization of partially purified S1 nuclease on Con A-Sepharose, made a similar observation. In the case of pea seed nuclease, which has two subunits, it has been suggested that the phosphomonoesterase activity resides in one of the subunits, while the nuclease activity requires both the subunits [88]. Chemical modification studies on purified S1 nuclease showed the involvement of single lysine and histidine residues in the catalytic activity of the enzyme. The substrates of S1 nuclease, ssDNA, RNA and 3′-AMP, protected the enzyme against 2,4,6-trinitrobenzenesulfonic acid (TNBS)-mediated inactivation, whereas this was not observed in the case of either methylene blue or diethylpyrocarbonate (DEP)-mediated inactivation of the enzyme. Moreover, the lysine (TNBS)-modified enzyme showed a significant decrease (70%) in its ability to bind 5′-AMP, a competitive inhibitor of S1 nuclease, while the histidine (DEP)-modified enzyme could effectively bind 5′-AMP, suggesting the involvement of lysine in substrate binding and histidine in catalysis. Furthermore, lysine and histidine modification was accompanied by a concomitant loss of ssDNase, RNase and phosphomonoesterase activities of the enzyme, indicating the existence of a common catalytic site for the hydrolysis of both monomeric and polymeric substrates [97, 290]. Similarly, studies on the active site nature of nuclease Bh1 revealed the involvement of a single lysine and carboxylate residue in the catalytic activity of the enzyme. Substrate protection and competitive inhibitor-binding studies on native, lysine- and carboxylate-modified enzyme suggested the involvement of lysine in substrate binding and carboxylate in catalysis. These studies also revealed that the hydrolysis of ssDNA, RNA and 3′-ribonucleotides occurs at a common catalytic site [291]
Substrate binding in P1 nuclease was studied by soaking the tetragonal crystals with single-stranded dithiophosphorylated R-stereoisomers of di-, tetra- and hexanucleotides as substrate analogues [292]. The studies revealed the presence of two nucleotide-binding sites, one at the active site close to the catalytic zinc and the second approximately 20 Å away at the periphery of the molecule. At both sites the base recognition involved stacking interactions with exposed aromatic residues as well as hydrogen-bonding contacts with the carboxylate groups. Thus, interaction occurred with Phe61 and Val132 at the first binding site and with Tyr144 and Tyr155 at the second site. However, the hydrogen bonding occurred between the adenine residues of the distereoisomer and Asp63 and Asp146 of the protein molecule [285]. Stacking interactions have been observed in many ssDNA and RNA binding proteins as well as nucleases of the Serratia family and this property can be considered as a hallmark of this class of proteins [293, 294, 213]. Similarly, differences in the contacts of the substrate with amino acid side chains in the protein were correlated to the observed differences in base specificity and pH optima for the hydrolysis of various substrates by P1 nuclease. Since no reports exist on site-directed mutagenesis of single-strand-specific nucleases, the exact residues involved in substrate binding and catalysis have not been delineated. However, for the Serratia endonuclease family, the active site is characterized by histidine, arginine, asparagine and glutamic acid and these residues are conserved in this family [170, 286].
In the case of structure-specific bacteriophage T5 nuclease, lysine has been implicated in catalysis. Crystal structures of three representative enzymes of this class of nucleases showed two divalent-metal-binding sites typically separated by 8–10 Å. Site-directed mutagenesis was used to investigate the role of three lysine residues (Lys83, Lys196, and Lys215) situated close to two metal-binding sites in bacteriophage T5 5′→3′-exonuclease. Neither Lys196 nor Lys215 were essential for either the exonuclease or the endonuclease activity but mutation of these residues increased the dissociation constant for the substrate from 5 nM to 200 nM (Lys196A) and 50 nM (Lys215A), indicating that they might be involved in substrate binding. Biochemical analysis demonstrated that Lys83 is essential for the exonucleolytic activity on ssDNA but not for the endonucleolytic cleavage of flap structures. Hence the authors suggested that Lys83 probably acts as a general base. Moreover, the enzyme exhibited different pH optima for both the endonuclease and exonuclease activities, suggesting that this multifunctional enzyme uses different mechanisms for both endonuclease and exonuclease activities [295]. In general, it can be assumed that for both single-strand-specific as well as non-specific nucleases, histidine and carboxylate are involved in catalysis, while lysine/arginine is involved in substrate binding.
12.3 Water-assisted metal ion catalysis
As mentioned above, the crystal structures of most metallonucleases show water molecules at the active site. It may be that the metal water cluster itself is a conserved structural element in these enzymes. The water molecule acts as a nucleophile and attacks the phosphodiester bond in concert with the metal ion and the surrounding amino acid side chains. An assisting function of amino acid side chains in either orienting or activating the attacking water molecule has been proposed for a number of zinc-dependent enzymes [296]. In the two-metal-ion mechanism proposed by Beese and Steitz [297] for the 3′→5′-exonuclease activity of E. coli polymerase I, catalytic RNA [298] and alkaline phosphatase [299], a free phosphate oxygen is replaced in the solvent molecule bridging the two metal ions. One of the metal ions activated the attacking nucleophile while the other stabilized the leaving O3′-oxyanion. A similar mechanism has been proposed for the two divalent-metal-binding sites situated in the active site of Taq 5′-nuclease [300]. In the case of 3′→5′-exonuclease activity of Pol I, it was shown that one metal ion could promote the formation of a hydroxide ion while the second stabilized the penta-coordinate transition state [301]. However, with T4 RNase H [302] and FEN-1 [303], site-directed mutagenesis revealed that only one metal site is required for catalysis and the other is involved in substrate binding. Furthermore, the metal sites in T5 exonuclease were too far apart to participate in the postulated two-metal mechanism (8.1 Å in T5 exonuclease versus 3.9 Å in 3′→5′-exonuclease of Pol I) [304, 295]. The conservation of metal ion–water cluster has also been shown for the Serratia family nucleases [286]. Most researchers studying Serratia endonuclease have proposed that DNA cleavage proceeds by attack of an active-site water molecule at the phosphorous atom of the bridging phosphate via phosphorane formation followed by cleavage of the 3′ O–P bond [305–307]. Miller et al. [286] proposed two schemes for the hydrolysis of phosphodiester bond by Serratia endonuclease: (i) an unligated water molecule may be directly activated by His89, or (ii) magnesium-bound water is activated by His89 wherein magnesium may alter the pKa of the bound water and produce a more nucleophilic hydroxide ligand, and this activated water molecule may mediate the hydrolysis of the phosphodiester bond.
In contrast to the two-metal-ion mechanism, Romier et al. [292] proposed a three-metal-ion mechanism to explain the action of P1 nuclease (Fig. 5). Accordingly, the scissile phosphate of the substrate sitting between the three zinc atoms binds close to ZnII, with its free oxygens replacing the two water molecules, and the base 5′ to the cleaved bond stacks against Phe61 and forms hydrogen-bonding contacts with Asp63. The water molecule bridging ZnI and ZnIII, which, as in other binuclear metallohydrolases, is presumably present as a hydroxide ion due to lowering of its pKa by the metal ions, acts as the nucleophile attacking the phosphate in-line with P–O3′ bond [308]. Asp45, which also serves as a ligand of ZnI, helps to properly orient the hydroxide for the attack. Arg48 stabilizes the resulting penta-coordinate transition state, and the attacking hydroxide ion together with the leaving oxyanion (O3′) occupy apical positions in this transition state. ZnII plays a crucial role in activating the phosphate and stabilizing the leaving O3′-oxyanion (Fig. 5). Thus all three of the zinc ions are important for catalysis [292].
Proposed catalytic mechanism of P1 nuclease. (Reprinted from Romier et al. [292], ©1998, with permission from Wiley–Liss Inc.)
S1 nuclease from A. oryzae is highly homologous to P1 nuclease (49.3% sequence identity), contains 3 g atoms of Zn2+ per mol of the enzyme and also requires Zn2+ for its activity. However, they differ in their pH optima and preference for various substrates. All the zinc ligands, as well as Phe61 and Asp63 at the active site-binding pocket are conserved in S1 nuclease. On the other hand, Arg48 in P1 nuclease is replaced by a lysine in S1 nuclease. Moreover, Gite et al. [97] have implicated lysine in substrate binding in S1 nuclease. Based on these observations, it can be assumed that the arginine in P1 nuclease and lysine in S1 nuclease have similar functions. However, Tyr144, Asp146 and Tyr155, which form the Tyr site in P1 nuclease, are replaced by Glu, Asn and Thr, respectively, in S1 nuclease. Although the structure of the P1 nuclease–ssDNA complex clearly demonstrated the role of the Tyr site in nucleotide binding, they fail to provide a convincing explanation for its general function. Studies on the crystal structure of S1 nuclease in the presence of its substrate analogs might help in evaluating the significance of this site in S1 nuclease [292].
13 Biological role
As mentioned earlier, nucleases play an important role in the four R's, i.e. replication, recombination, restriction and repair. The importance of DNA nicking in recombination is strongly suggested by the formation of nicks or gaps in DNA during meiotic prophase, essential for preparing the substrates for the formation of DNA heteroduplexes. Nucleases have been shown to play an important role in the formation of nicks during meiotic recombination. Holloman and Holiday [142] observed that certain mutant strains of U. maydis, unable to carry out allelic recombination, showed reduced nuclease levels and hence suggested its role in recombination. High levels of endonuclease activity were observed in the basidiocarp of C. cinereus during the late S-phase and early karyogamy [309]. The authors also demonstrated that cofactors, such as Mg2+ and Ca2+ or high temperatures, enhanced the nicking activity during these meiotic phases, leading to increased frequency of recombination. In contrast, DNA polymerase b peaked at late pachytene but the nuclease levels were low. Both the endonuclease and the polymerase activities were shown to be essential for meiotic recombination in C. cinereus[310]. The last step in recombination is repair of nicks and gaps and at times may contain mismatched base pairs that are corrected by excision repair. The low levels of Ustilago nuclease in the recombination deficient mutants suggested its role in excision repair [142]. Studies on nuclease α from U. maydis showed that it could recognize base mismatches and cleave the heteroduplexes, pointing towards its role in mismatch repair [27]. In the case of N. crassa compared with a wild strain, the repair-deficient and UV-sensitive mutants (uvs-2, uvs-3, uvs-6 and nuh-4) could not secrete endo-exonucleases. In addition, these mutants had a higher level of endo-exonuclease precursor than the wild-type, indicating that these mutants may have some defect either in the protease(s) that control the nuclease level or in the regulation of protease(s). The above mutants were also sensitive to various mutagens and mitomycin C, and exhibited a high frequency of spontaneous, recessive lethal mutations and deletions, indicating the involvement of N. crassa nuclease in repair [107]. Furthermore, the ability of S1, Ustilago, and Alteromonas nucleases to recognize minor distortions in DNA brought about by UV-irradiation, apurinization or carcinogenic and mutagenic agents point towards their probable role in DNA repair [9]. The branched DNA structures formed during replication, recombination and repair are recognized and cleaved by 5′→3′-exonucleases. The DNA strand displaced from the site of a nick by DNA polymerase during nick translation gives rise to a flap structure which is cleaved by these structure-specific nucleases. Okazaki fragments on the lagging strand formed during replication yield similar structures in which the displaced 5′-end consists of short stretches of ribonucleotides that form the primers for lagging-strand synthesis. These structures are also cleaved by the 5′→3′-exonuclease activity [267]. Nucleases have also been implicated in the restriction of invading pathogens by degrading the incoming nucleic acids. For example, nucleases from S. antibioticus and S. glaucescens circumstantially restrict the growth of actinophages [25, 24, 311].
An important role of the extracellular nucleases is scavenging of nucleosides and phosphate for growth. Such enzymes exhibit an associated phosphomonoesterase activity. The role of nucleases in nutrition has been demonstrated in the trypanosome C. luciliae[135]. Nucleases from S. antibioticus and S. glaucescens were shown to play an important role in the recycling of nucleotides from the substrate mycelium to aerial mycelium [312, 154, 155]. Recently, Nicieza et al. [313] isolated two extracellular nucleases from S. antibioticus, with an Mr of 18 and 34 kDa, which were nutritionally regulated and reached their maximum activity during aerial mycelium formation and sporulation. Their role appeared to be DNA degradation in the substrate mycelium and supply of building blocks for macromolecular biosynthesis in aerial mycelium and they acted in concert with the periplasmic nuclease. Of the two extracellular nucleases, the 18-kDa nuclease appeared to be reminiscent of NUC-18, a thymocyte nuclease proposed to have a key role in glucocorticoid-stimulated apoptosis [314, 315]. The Streptomyces 18-kDa nuclease showed a requirement of both Mg2+ and Ca2+ and, like NUC-18, was inhibited by Zn2+ and aurintricarboxylic acid. Interestingly, the N-terminal sequence of the 18-kDa protein showed striking similarity to proteins of the cyclophilin family [316]. Native cyclophilins also degrade DNA in a Ca2+/Mg2+-dependent manner and their role in apoptosis has been reviewed by Montague et al. [317].
Programmed cell death, or apoptosis, is a phenomenon occurring universally in all unicellular and multicellular organisms [318–320]. Its regulation is essential for the normal development as it serves to remove surplus cells and virally infected or tumor cells [321–323]. An early biochemical change recognized as a hallmark of apoptosis is internucleosomal DNA cleavage. Recently, the generation of large DNA fragments (∼30–50 kb) and single-strand nicking have also been reported to be associated with apoptosis. Several endonucleases, such as DNase I, DNase II, NUC18, NUC58, NUC40 and 27- and 37-kDa endonucleases, have been suggested as enzymes responsible for this characteristic DNA cleavage [324–327]. Most of the nucleases having a role in apoptosis can efficiently degrade double-stranded nucleic acids, and are Ca2+/Mg2+-dependent enzymes that produce 3′-OH DNA breaks. However, they are inhibited by Zn2+ and aurintricarboxylic acid.
DNA/RNA non-specific nucleases like those from S. aureus and S. epidermidis are found in a variety of clinical and food infections [328–333]. Similarly, S. marcescens nuclease [334] has been postulated to play a role during invasion or establishment of an infection. However, the role of single-strand-specific nucleases in diseases has not been postulated. Bufe et al. [335, 336] discovered that the major group V allergen of grass pollen, Phleum pratense 5b (Phlp5b) from timothy grass, showed ribonuclease activity. Subsequently, Matousek et al. [337] showed that pollen RNases, owing to their ability to degrade dsRNA, may function as defense proteins against viral infection, as components of a degradation complex which participates in the apoptosis of the tapetal cell layer and for nucleoside re-utilization by the developing pollen. However, the detailed characterization of Phlp5b nuclease indicated that it is specific for ssDNA and RNA. Interestingly, the enzyme did not degrade dsDNA but showed functional characteristics of a topoisomerase [338]. Phlp5b is located in the cytosol [339] and is quickly released from the pollen grain once the cell is humidified [340]. The interesting feature of this allergen is that the C-terminal 13-kDa component showed significantly higher nuclease activity than the full-length holoallergen as seen from the crystallization studies. Moreover, studies on the active-site nature revealed the involvement of two tyrosine residues and a region of four amino acids strikingly similar to those from the active-site region of topoisomerase I from E. coli[341]. Fusarium solani f. spp. pisi is a pathogen of pea (Pisum sativum) while Fusarium solani f. spp phaseoli is a pathogen of bean (Phaseolus vulgaris). These species produced a heat-stable nuclease, activated by Mn2+ and Ca2+, and a marked stimulation of nuclease production occurred during macroconidium germination on pea pod endo-carp surfaces [342]. Hence, the authors suggested that the nuclease production by these species is stimulated by the host and might contribute to the virulence towards host plants.
14 Applications
14.1 Analytical
Since their discovery, single-strand-specific nucleases have been extensively used as analytical tools for the determination of nucleic acid structure, owing to their ability to recognize single-stranded nucleic acids and a wide variety of nucleic acid structures as well as structural variations. S1 nuclease has been the most widely used enzyme in molecular biology research. Its high selectivity for single-stranded nucleic acids has been employed for the estimation of double-helical content of various single-stranded nucleic acids [343] and for the isolation and characterization of double-stranded regions of single-stranded nucleic acids [344–346]. It has also been used for the removal of ssDNA in genetic manipulation, S1 mapping to determine transcriptional initiation sites [347, 348], and two-dimensional analysis of complexible transcripts [347, 349–351]. A variety of methods was developed to screen genetic mutations using single-strand-specific nucleases [352–354]. Due to the ability of S1 nuclease to cleave basepair mismatches in DNA/DNA heteroduplexes, it was used for heteroduplex analysis of PCR products [355]. Moreover, S1, N. crassa and BAl 31 nucleases can recognize structural alterations induced by various mutagenic agents and introduce single- and double-stranded breaks in DNA and hence can be used for the detection of locally altered structures in DNA [9]. Hatakeyama et al. [356] immobilized the DNA probe on latex particles and noted that DNAs containing single-point mutations failed to bind strongly to the immobilized probe, as opposed to native DNAs. The ability of S1 nuclease to cleave unhybridized and loosely hybridized DNA regions on the probe was exploited for the detection of fully complementary hybrids. Some other applications of S1 nuclease include the removal of single-stranded tails prior to DNA ligation [194], isolation of inserts from plasmid DNA [357], screening of DNA binding proteins and substances [358, 359], study of palindromic sequences in DNA [360], enzymatic synthesis of globin genes in vitro [361] and detection of non-B secondary structures [362]. S1 nuclease was used for locating the 5′- and 3′-termini of mRNA and DNA templates, the 5′- and 3′-splice junctions in relation to sites of cleavage with restriction enzymes in cloned genes or double-stranded cDNA and to quantitate the amount of specific classes of mRNA in RNAs extracted from tissues or cultured cells [363]. It has also been found useful for the structural analysis of tRNAs and rRNAs [224]. The application of S1 nuclease for the analysis of RNA has been extensively reviewed by Lefebvre and Viville [364]. Similarly, plant nucleases such as the rye germ nuclease [365–367] and wheat chloroplast nuclease in combination with rye germ nuclease [368, 369] were used for the structural determination of various tRNAs.
P1 nuclease from P. citrinum has been used for the isolation of eukaryotic mRNA cap structure [370], base composition analysis of nucleic acids [371], removal of nucleic acids during protein purification [372], sequence analysis of end-group-labeled RNA [373] and analysis of tRNA structure [374].
The N. crassa nuclease was employed for the isolation of pure lac operon [375], isolation of tRNA and rRNA gene hybrids [376, 377] and for the detection of sequence heterology [378]. BAL 31 nuclease shows exonuclease activity and shortens duplex DNA from both 3′- and 5′-ends. This property was exploited for ordering restriction endonuclease-generated DNA fragments [379].
14.2 Industrial
Monosodium glutamate has long been used as a food-flavoring agent. However, the realization that the addition of an equimolar mixture of 5′-GMP and 5′-IMP to monosodium glutamate can significantly increase the flavor-enhancing capacity has led to considerable interest in the production of 5′-mononucleotides. Additionally, the derivatives of IMP and GMP, like 2-methylinosine-5′-monophosphate, 2-N-methylguanosine-5′-monophosphate and 2-N,N-dimethylguanosine-5′-monophosphate, when fortified with l-glutamic acid or l-homocysteic acid, not only act as strong flavor enhancers but also resist the deterioration effect of the enzymes present in food [380]. P1 nuclease from P. citrinum, which degrades RNA to 5′-mononucleotides, has been used for the industrial production of 5′-mononucleotides from yeast RNA [15]. Sheep-kidney nuclease which converts single-stranded nucleic acid to 5′-dinucleotides and trinucleotides as the final products, can also be used for the production of all the deoxydinucleotides [381].
15 Future perspectives
Nucleases take part in a variety of cellular events associated with the transfer and maintenance of genetic material. Due to their ability to recognize a wide variety of nucleic acid structures, considerable efforts have been made to evaluate the role of nucleases in different cellular processes as well as their application as analytical tools to study nucleic acids structure. Among them, the utility of single-strand-specific nucleases as analytical tools has been widely recognized and has led to their extensive application as probes for the determination of nucleic acid structure. The majority of these enzymes share common properties, such as multiple activities, metal ion requirements, their metalloprotein and glycoprotein nature. Since single-strand-specific nucleases have been used mainly as analytical tools, very little attention was paid to their chemical nature, structure–function relationships and biological roles. However, the amino-acid sequences of S1 and P1 nuclease revealed that there is approximately 50% sequence similarity between them. The comparison of primary sequences, conserved sequences and residues present at the active site of single-strand-specific nucleases will advance our understanding on evolutionary aspects of these enzymes. Moreover, detection, purification and extensive characterization of single-strand-specific nucleases, which exhibit pH optima around neutrality and do not require metal ions for their activity, will not only provide a convenient probe for studying nucleic acid structure but also advance our knowledge on the physicochemical characteristics and biological roles of this class of enzymes.
Acknowledgements
The authors thank Nitin Patil for his help in the preparation of the manuscript. The work was supported by a grant from the Department of Science and Technology, Government of India, to V.S. This is communication no. 6631 from the National Chemical Laboratory, Pune.
References
Laskowski, M., Sr. (1985) Nucleases: historical perspectives. In: Nucleases (Linn S.M. and Roberts, R.J., Eds.), pp1–21. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Shishido, K. and Ando, T. (1985) Single-strand-specific nucleases. In: Nucleases (Linn S.M. and Roberts, R.J., Eds.), pp. 155–185. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Sadowski, P.D. (1985) Role of nucleases in genetic recombination. In: Nucleases (Linn S.M. and Roberts, R.J., Eds.), pp. 23–40. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Linn, S. (1985) Nucleases involved in DNA repair. In: Nucleases (Linn S.M. and Roberts, R.J., Eds.), pp. 59–83. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Brown, D.R., Hurwitz, J., Reinberg, D. and Zipursky, S.L. (1985) Nuclease activities involved in DNA replication. In: Nucleases (Linn S.M. and Roberts, R.J., Eds.), pp. 187–209. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Mishra, N.C. (1995) In Molecular Biology of Nucleases (Mishra, N.C., Ed.), pp. 1–21. CRC Boca Raton, FL.
Umemura, K., Nagami, F., Okada, T., Kuroda, R. AFM characterization of single-strand-specific endonuclease activity on linear DNA,. Nucleic Acids Res. 28, 2000. E39
Gray, H.B., Jr., Winston, T.P., Hodnett, J.L., Legerski, R.J., Nees, D.W., Wei, C.F. and Robberson, D.L. (1981) The extracellular nuclease from Alteromonas espejiana: an enzyme highly specific for nonduplex structure in nominally duplex DNAs. In: Gene Amplification and Analysis, Vol. 2 (Chirikijian, J.G. and Papas, T.S., Eds.), pp. 169–203. Elsevier, North Holland/New York.
Fraser, M.J. and Low, R.L. (1993) Fungal and mitochondrial nucleases. In: Nucleases (Linn, S.M., Lloyd, R.S. and Roberts, R.J., Eds.), pp. 171–207. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Rushizky, G.W. (1981) S1 nuclease of Aspergillus oryzae. In: Gene Amplification and analysis, Vol. 2 (Chirikjian, J.G. and Papas, T.S., Eds.), pp. 205–215. Elsevier, North Holland/New York.
Liou, F.C., Lin, C.M. and Hsieh, W.T. (1986) Purification and characterization of nucleases S1 from Aspergillus oryzae. J. Chin. Biochem. Soc. 15, 39–49 (Chem. Abstr. 108:90477).
Desai, N.A. (2001) Extracellular Nuclease from Basidiobolus haptosporus (89-3-24): Purification and Characterization. Ph.D. dissertation, Pune.
Gonikberg, E.M. (1978) Cleavage of bacteriophage PM2 DNA by nuclease S1. Biokhimiya (Moscow) 7, 1285–1293 (Chem. Abstr. 89:159346).
Cotton, F.A. and Hazen, E.E., Jr. (1971) Staphylococcal nuclease: X-ray structure. In: The Enzymes, Vol. 3 (Boyer, P.D., Ed.), pp. 153–170. Academic Press, New York.
Bauer, W. and Vinograd, J. (1974) Circular DNAs. In: Basic Principles of Nucleic Acid Chemistry, Vol. 2 (Cantoni, G.L. and Davis, D.R. Eds.), pp. 265–303, Academic Press, New York.
Wells, R.D., Goodman, T.C., Hillen, W., Horn, G.T., Klein, R.D., Larson, J.E., Müller, U.R., Neuendorf, S.K., Panayotatos, N. and Stirdivant S.M. (1980) DNA structure and gene regulation. In: Prog. Nucleic Acids Res. Mol. Biol., Vol. 24 (Cohn, W.E., Ed.), pp. 167–267.
Coen, E., Robbins, T.P., Almeida, J., Hudson, A. and Carpenter, R. (1989) Consequences and mechanisms of transposition in Antirrhinum majus. In: Mobile DNA (Berg, D.E. and Howe, M.M., Eds.), pp. 413–436. ASM Press, Washington DC.
Anfinsen, C.B., Cuatrecasas, P. and Taniuchi, H. (1971) Staphylococcal nuclease: chemical properties and catalysis. In: The Enzymes, Vol. 4 (Boyer, P.D., Ed), pp. 177–204. Academic Press, London.
Kostriken, R., Strathern, J., Klar. Amar J.S., Hicks, J.B., Moomaw, C. and Heffron, F. (1983) A site-specific endonuclease from Saccharomyces cerevisiae which plays an essential role in mating type interconversion. UCLA Symp. Mol. Cell. Biol., New Ser. 10, 785–795 (Chem. Abstr. 100:187876).
Andrews, J., Martin-Bertram, H. and Hagen, U. (1984) S1 nuclease-sensitive sites in yeast DNA: an assay for radiation-induced base damage. Int. J. Radiat. Biol. Relat. Stud. Phys., Chem. Med. 45, 497–504 (Chem. Abstr. 101:68499).
Suck, D. (1992) Recognition of double- and single-stranded substrates by nucleases: structural studies of DNase I and P1 nuclease. In: Molecular Structure and Life (Kyogozu, Y. and Nishimura, Y., Eds.), pp. 141–156. CRC Press, Boca Raton, FL.
White, E. (1996) Life, death, and the pursuit of apoptosis. Genes Dev. 10, 1–15 (Chem. Abstr. 124:107758).
Huang, S.-T., Hughes, F.M., Jr. and Cidlowski, J.A. (1997) Candidate nuclease responsible for genomic degradation during apoptosis. Comments Toxicol. 5, 555–569 (Chem. Abstr. 127:93039).
Famulski, K.S., Macdonald, D., Paterson, M.C. and Sikora, E. (1999) Activation of a low pH-dependent nuclease by apoptotic agents. Cell Death Differ. 6, 281–289 (Chem. Abstr. 131:85942).
Sachs, D.H., Berzofsky, J.A., Pisetsky, D.S. and Schwartz, R.H. (1978) Genetic control of the immune response to Staphylococcal nuclease. Springer Semin. Immunopathol. 1, 51–83 (Chem. Abstr. 89:105550).
Gudding, R. (1980) Measurement of Staphylcoccus aureus nuclease and antinucleases: applicability for the assessment of mastitic milk. Acta Vet. Scand. 21, 229–241 (Chem. Abstr. 93:163241).
Gudding, R. (1980) Nuclease of Staphylococcus epidermidis isolated from mastitic milk: production and some properties. Acta Vet. Scand. 21, 267–277 (Chem. Abstr. 93: 110305).
Sundaram, S.P., Kumari, S.L.S. and Murthy, K.V. (1982) Thermostable nuclease activity of Staphylococci from clinical sources. Indian J. Med. Res. 75, 19–22 (Chem. Abstr. 96:177612).
Stersky, A.K., Szabo, R., Todd, E.C.D., Thacker, C., Dickie, N. and Akhtar, M. (1986) Staphylococcus aureus growth and thermostable nuclease and enterotoxin production in canned salmon and sardines. J. Food Prot. 49, 428–435 (Chem. Abstr. 105:77691).
Nunez, M., Bautista, L., Medina, M., and Gaya, P. (1988) Staphylococcus aureus, thermostable nuclease and Staphylococcal enterotoxins in raw ewes′ milk Manchego cheese. J. Appl. Bacteriol. 65, 29–34 (Chem. Abstr. 110:6595).
Hejazi, A. and Falkiner, F.R. (1997) Serratia marcescens. J. Med. Microbiol. 46, 903–912 (Chem. Abstr. 128:20317).
Bufe, A., Becker, W.-M., Schramm, G., Petersen, G., Petersen, A., Mamat, U. and Schlaak, M. (1994) Major allergen ph1 p Va (timothy grass) bears at least two different Ig E-reactive epitopes. J. Allergy Clin. Immunol. 94, 173–181 (Chem. Abstr. 124:115308).
Vrtala, S., Grote, M., Duchene, M., van Ree, R., Kraft, D., Scheiner, O. and Valenta, R. (1993) Properties of tree and grass pollen allergens: reinvestigation of the linkage between solubility and allergenicity. Int. Arch. Allergy Immunol. 102, 160–169 (Chem. Abstr. 120:104869).
Hatakeyama, M., Nakamura, K., Iwato, S., Handa, H., Fujimoto, K. and Kawaguchi, H. (1998) DNA-carrying latex particles for DNA diagnosis 2. Distinction of normal and point mutant DNA using S1 nuclease. Colloids Surf. B. Biointerfaces 10, 171–175 (Chem. Abstr. 128:279295).
Lefebvre, L. and Viville, S. (1998) S1 nuclease analysis of RNA. In: Molecular Biomethods Handbook (Rapley, R. and Walker, J.M., Eds.), pp. 35–49. Humana Press, Totowa, NJ.
Gutcho, S. (1970) Nucleotides as flavor enhances. In: Nucleotides and Nucleosides (Gutcho, S., Ed.), pp. 181–195. Noyes Data Corporation, NJ.
Author notes
Present-address: Department of Biochemistry, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, New York, NY 10461, USA.



![DNA configurations recognized by five different nucleases [B, bond attacked; S, structure attacked; P, preferred conformation]. a: DNAase I: B: O-3′-P; S: two sugar–phosphate strands that are closely spaced; P: minor groove of duplex. b: S1 nuclease: B: O-3′-P; S: exposed single-strand; P: ends of duplex, tip of loop. c: Microccal nuclease: B: O-5′-P; S: exposed single-strand, unpaired A or T base. P: 5′-end of duplex, side of loop, weakly in duplex. d: Copper–phenanthroline: B: sugar ring; S: basepair step; P: duplex. e: DNase II: B: O-5′-P; S: slightly exposed, stacked single-strand; P: duplex with wide minor groove, stacked loop. Reprinted with permission from Drew [10].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/26/5/10.1111_j.1574-6976.2003.tb00626.x/1/m_FMR_457_f1.jpeg?Expires=1716413513&Signature=ClPvFgbb~620EUvi7NHz6x33q49qA6CfQTzyvaJG9lEAnqK4qvSxYAABAqjW70Yex189js364KRsPb5i6jtVRnxQZ2w04fIB9ejP4Ey-0~B-Rey0meSeRuWuFmQvLTQQFSQoAHJwgB3OCdYeDTgOWDaGQIzNTOyg6-n-Zu3Vz5vUdDqzvRzpATRwrEOxLd3hh5WucgIiVhsujotmHtr4ZIRB6fnMEmZ9XdIPQMuzB2r3CfMZuppUp9UcnHq-GpqfIkLD24zLRuYWYCAIvzZPmZxlcFjPBjr5LA7~3Ereb-J41BNUdE7pmIngwfOBB5vaqc0kqnOsgO4EkBCRf~IEBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![A: Substrates of 5′→3′-exonucleases. B: Action of 5′-nuclease on flap structure during nick translation. The invading primer is shown in bold line. It is extended by the action of Pol I and the displaced strand forms a flap structure. The endonucleolytic cleavage by 5′-nuclease is indicated by a bold arrow, whereas the faded arrow indicates exonucleolytic attack. (Reprinted from Ceska and Sayers, [267], ©1998, with permission from Elsevier Science.)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/26/5/10.1111_j.1574-6976.2003.tb00626.x/1/m_FMR_457_f2.jpeg?Expires=1716413513&Signature=OB4aXsj-mj0dGu8KM-dMSyFPmO~x4ruWG77gJbGnZjvd0d0K2Uuou3K7FptTQgFNKiU21vGoOzblVP1qWyVgjleGWOD9lZBJxMXzD7FEZln0HhKc2SkOK-4QLFeeM3u7itBjVm6XSUXWrXi6UFCbLdVXYiIVU4ergJVm6QmoZrssQdk9CgMyiKkwit1S5AAhVnshVkgF9zjlN~~nXLduOl6LLOmq9YX-LQxz-xmxC3Gi0dtKkxHWi1gYUOwogFGvmg3nr3yWP46~60Za04cZ9bU40xh0l20y~oWcY~HTqwMm-UdpgazfrN37Yc4MQwegzdUAYzKx7LF8T1ueVTdBQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
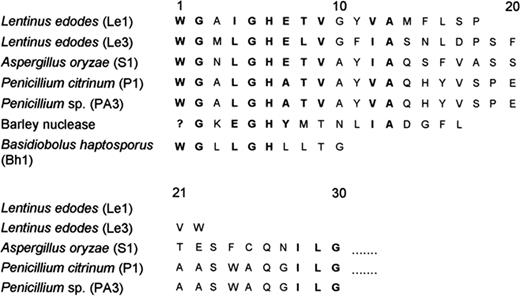
![Coordination of zinc ions in P1 nuclease. 01, 02, 03 represent the water ligands. (Reprinted from Volbeda et al. [281], ©1991, with permission from Oxford University Press.)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/26/5/10.1111_j.1574-6976.2003.tb00626.x/1/m_FMR_457_f4.jpeg?Expires=1716413513&Signature=QVn6l1U8cNQgpiMps1C0X83le7xndbdak424QWPVt5UVJwnERn~ejtCqoYiLMqUWxxLQyMDzT9SALby-DdJ0buQirD1ZHGTYJryWvDguig8U66pM-Jjb9dBGwE4jZ6BvIr7oNN-JkajGh9bYuwtqeUj1swGAbsQ~VZcpUGgtcV55RfAO3XG9XHAkE-WyRVGIPiVoH~rZD5uVaUiYEp7TzplY029wlK9AbiZJyc~p0M0E8edBfKJ7VrY4e5igAYg8DBzhfGQeB8DgRJPR-YswXT-~p5-s~fU5AzfKUUZYprEtNtswNrnoY4BMoLx~f-7whwEFl-fh~N9DPyL9VHJeZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Proposed catalytic mechanism of P1 nuclease. (Reprinted from Romier et al. [292], ©1998, with permission from Wiley–Liss Inc.)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsre/26/5/10.1111_j.1574-6976.2003.tb00626.x/1/m_FMR_457_f5.jpeg?Expires=1716413513&Signature=mCGOr-8rLXyzzfM6U3pG1L2nI1MHNDNpFOQ0v5TCScFng6a15EkjMfTSyj70F0sBuTQgLD30zAHHB4pqdbuYqQ7e1lVboLDuOcAZfmdsI6kiFPpMFc1e2e2Z8vslCODoMwKRxHYh1ZtNCI4ZcOfB5z0Yy7PpnOOucfFFh0prK-tMAw8qDVLeAWH2ZO5hoJq~Y3kDCpUyqgaxYCZthaYfqO~OKsX1pLKeHqLCSo9~orSPPdh3QEg0uUlU8U-Tw7-OIVzchcSWBw7CHenhoazGw11EhzkRaWkfHwHs4TqcDZLPORqVN6FGX3NQbrsWmkff2T5v~uKm17Z7vQynsaZJyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
